Abstract
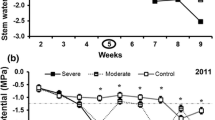
Drought can alter plant quality and the strength of trophic interactions between herbivore groups, and is likely to increase in occurrence and severity under climate change. We hypothesized that changes in plant chemistry due to root herbivory and drought stress would affect the performance of a generalist and a specialist aphid species feeding on a Brassica plant. High drought stress increased the negative effect of root herbivory on the performance of both aphid species (30 % decrease in fecundity and 15 % reduction in intrinsic rate of increase). Aphid performance was greatest at moderate drought stress, though the two species differed in which treatment combination maximized performance. Nitrogen concentration was greatest in high and moderately drought-stressed plants without root herbivores and moderately drought-stressed plants under low root herbivore density, and correlated positively with aphid fecundity for both species. Glucosinolate concentrations increased 62 % under combined drought stress and root herbivory, and were positively correlated with extended aphid development time. Root herbivory did not influence relative water content and foliar biomass under normal water regimes but they decreased 24 and 63 %, respectively, under high drought stress. This study shows that drought can alter the strength of interactions between foliar and root herbivores, and that plant chemistry is key in mediating such interactions. The two aphid species responded in a broadly similar way to root herbivore and drought-stress treatments, which suggests that generalized predictions of the effects of abiotic factors on interactions between above- and below-ground species may be possible.






Similar content being viewed by others
References
Agerbirk N, De Vos M, Kim J, Jander G (2009) Indole glucosinolate breakdown and its biological effects. Phytochem Rev 8:101–120. doi:10.1007/s11101-008-9098-0
Agrawal AA, Fishbein M (2006) Plant defense syndromes. Ecology 87:132–149. doi:10.1890/0012-9658
Ahuja I, Rohloff J, Bones AM (2010) Defence mechanisms of Brassicaceae: implications for plant-insect interactions and potential for integrated pest management. A review. Agron Sustain Dev 30:311–348. doi:10.1051/agro/2009025
Bale JS, Masters GJ, Hodkinson ID, Awmack C, Bezemer TM, Brown VK, Butterfield J, Buse A, Coulson JC, Farrar J, Good JEG, Harrington R, Hartley S, Jones TH, Lindroth RL, Press MC, Symrnioudis I, Watt AD, Whittaker JB (2002) Herbivory in global climate change research: direct effects of rising temperature on insect herbivores. Glob Change Biol 8:1–16. doi:10.1046/j.1365-2486.2002.00451.x
Bezemer TM, Jones TH (1998) Plant-insect herbivore interactions in elevated atmospheric CO2: quantitative analyses and guild effects. Oikos 82:212–222
Bezemer TM, Wagenaar R, van Dam NM, Wäckers FL (2003) Interactions between above- and belowground insect herbivores as mediated by the plant defense system. Oikos 101:555–562. doi:10.1034/j.1600-0706.2003.12424.x
Blande JD, Pickett JA, Poppy GM (2007) A comparison of semiochemically mediated interactions involving specialist and generalist Brassica-feeding aphids and the braconid parasitoid Diaeretiella rapae. J Chem Ecol 33:767–779. doi:10.1007/s10886-007-9264-7
Cole RA (1997) The relative importance of glucosinolates and amino acids to the development of two aphid pests Brevicoryne brassicae and Myzus persicae on wild and cultivated Brassica species. Entomol Exp Appl 85:121–133. doi:10.1046/j.1570-7458.1997.00242.x
Crawley MJ (2007) The R book. Wiley, West Sussex
Douglas AE (2006) Phloem-sap feeding by animals: problems and solutions. J Exp Bot 57:747–754. doi:10.1093/jxb/erj067
Finch S, Coaker TH (1969) A method for the continuous rearing of the cabbage root fly Erioischia brassicae (Bch.) and some observations on its biology. Bull Entomol Res 58:619–627. doi:10.1017/S0007485300057345
Finch S, Jones TH (1989) An analysis of the deterrent effect of aphids on cabbage root fly (Delia radicum) egg-laying. Ecol Entomol 14:387–391. doi:10.1111/j.1365-2311.1989.tb00940.x
Gange AC, Brown VK (1989) Effects of root herbivory by an insect on a foliar-feeding species, mediated through changes in the host plant. Oecologia 81:38–42
Gols R, Bukovinszky T, van Dam N, Dicke M, Bullock J, Harvey J (2008) Performance of generalist and specialist herbivores and their endoparasitoids differs on cultivated and wild brassica populations. J Chem Ecol 34:132–143
Grace J (1997) Plant water relations. In: Crawley MJ (ed) Plant ecology. Blackwell, Oxford, pp 28–50
Hartley SE, Jones TH (2003) Plant diversity and insect herbivores: effects of environmental change in contrasting model systems. Oikos 101:6–17. doi:10.1034/j.1600-0706.2003.12566.x
Heaney RK, Spinks EA, Hanley AB, Fenwick GR (1986) Technical bulletin: analysis of glucosinolates in rapeseed. AFRC, Food Research Institute, Norwich, pp 1–25
Hopkins RJ, van Dam NM, van Loon JJA (2009) Role of glucosinolates in insect-plant relationships and multitrophic interactions. Annu Rev Entomol 54:57–83. doi:10.1146/annurev.ento.54.110807.090623
Jander G, Howe G (2008) Plant interactions with arthropod herbivores: state of the field. Plant Physiol 146:801–803. doi:10.1104/pp.104.900247
Johnson SN, Staley JT, McLeod FAL, Hartley SE (2011) Plant-mediated effects of soil invertebrates and summer drought on above-ground multitrophic interactions. J Ecol 99:57–65. doi:10.1111/j.1365-2745.2010.01748.x
Jones CG, Hartley SE (1999) A protein competition model of phenolic allocation. Oikos 86:27–44. doi:10.2307/3546567
Kallis G (2008) Droughts. Annu Rev Environ Resour 33:85–118. doi:10.1146/annurev.environ.33.081307.123117
Kaplan I, Halitschke R, Kessler A, Rehill BJ, Sardanelli S, Denno RF (2008) Physiological integration of roots and shoots in plant defense strategies links above- and belowground herbivory. Ecol Lett 11:841–851. doi:10.1111/j.1461-0248.2008.01200.x
Kazana E, Pope TW, Tibbles L, Bridges M, Pickett JA, Bones AM, Powell G, Rossiter JT (2007) The cabbage aphid: a walking mustard oil bomb. Proc R Soc B 274:2271–2277. doi:10.1098/rspb.2007.0237
Kim JH, Jander G (2007) Myzus persicae (green peach aphid) feeding on Arabidopsis induces the formation of a deterrent indole glucosinolate. Plant J 49:1008–1019. doi:10.1111/j.1365-313X.2006.03019.x
King C, Jacob HS, Berlandier F (2006) The influence of water deficiency on the relationship between canola (Brassica napus L.), and two aphid species (Hemiptera: Aphididae), Lipaphis erysimi (Kaltenbach) and Brevicoryne brassicae (L.). Aust J Agric Res 57:439–445. doi:10.1071/ar05137
Kuśnierczyk A, Winge P, Jørstad TS, Troczyńska J, Rossiter JT, Bones AM (2008) Towards global understanding of plant defence against aphids—timing and dynamics of early Arabidopsis defence responses to cabbage aphid (Brevicoryne brassicae) attack. Plant Cell Environ 31:1097–1115. doi:10.1111/j.1365-3040.2008.01823.x
Larsson S (1989) Stressful times for the plant stress—insect performance hypothesis. Oikos 56:277–283
Leather S (1989) Do alate aphids produce fitter offspring—the influence of maternal rearing history and morph on life-history parameters of Rhopalosiphum padi (L). Funct Ecol 3:237–244
Masters GJ, Brown VK, Gange AC (1993) Plant mediated interactions between aboveground and belowground insect herbivores. Oikos 66:148–151
Newton E, Bullock J, Hodgson D (2009) Glucosinolate polymorphism in wild cabbage (Brassica oleracea) influences the structure of herbivore communities. Oecologia 160:63–76. doi:10.1007/s00442-009-1281-5
Newton E, Bullock J, Hodgson D (2010) Temporal consistency in herbivore responses to glucosinolate polymorphism in populations of wild cabbage (Brassica oleracea). Oecologia 164:689–699. doi:10.1007/s00442-010-1702-5
Radovich TJK, Kleinhenz MD, Streeter JG (2005) Irrigation timing relative to head development influences yield components, sugar levels, and glucosinolate concentrations in cabbage. J Am Soc Hort Sci 130:943–949
Schreiner M, Beyene B, Krumbein A, Stutzel H (2009) Ontogenetic changes of 2-Propenyl and 3-Indolylmethyl glucosinolates in Brassica carinata leaves as affected by water supply. J Agric Food Chem 57:7259–7263. doi:10.1021/jf901076h
Scriber JM, Slansky F (1981) The nutritional ecology of immature insects. Annu Rev Entomol 26:183–211. doi:10.1146/annurev.en.26.010181.001151
Slansky F, Wheeler GS (1992) Caterpillars compensatory feeding response to diluted nutrients leads to toxic allelochemical dose. Entomol Exp Appl 65:171–186. doi:10.1111/j.1570-7458.1992.tb01641.x
Soler R, Bezemer TM, van Der Putten WH, Vet LE, Harvey JA (2005) Root herbivore effects on above-ground herbivore, parasitoid and hyperparasitoid performance via changes in plant quality. J Anim Ecol 74:1121–1130. doi:10.1111/j.1365-2656.2005.01006.x
Staley JT, Mortimer SR, Morecroft MD, Brown VK, Masters GJ (2007) Summer drought alters plant-mediated competition between foliar- and root-feeding insects. Glob Change Biol 13:866–877. doi:10.1111/j.1365-2486.2007.01338.x
Staley JT, Mortimer SR, Morecroft MD (2008) Drought impacts on above-belowground interactions: do effects differ between annual and perennial host species? Basic Appl Ecol 9:673–681. doi:10.1016/j.baac.2007.10.006
Tariq M, Staley JT, Wright DJ (2010) Maternal host plant effects on aphid performance: contrasts between a generalist and a specialist species on Brussels sprout cultivars. Agric For Entomol 12:107–112. doi:10.1111/j.1461-9563.2009.00458.x
Tariq M, Wright DJ, Rossiter JT, Staley JT (2012) Aphids in a changing world: testing the plant stress, plant vigour and pulsed stress hypotheses. Agric For Entomol 14:177–185. doi:10.1111/j.1461-9563.2011.00557.x
R Development Core Team (2011) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
van Dam NM, Raaijmakers CE (2006) Local and systemic induced responses to cabbage root fly larvae (Delia radicum) in Brassica nigra and Brassica oleracea. Chemoecology 16:17–24. doi:10.1007/s00049-005-0323-7
van Dam NM, Raaijmakers CE, van der Putten WH (2005) Root herbivory reduces growth and survival of the shoot feeding specialist Pieris rapae on Brassica nigra. Entomol Exp Appl 115:161–170. doi:10.1111/j.1570-7458.2005.00241.x
van der Putten WH, Macel M, Visser ME (2010) Predicting species distribution and abundance responses to climate change: why it is essential to include biotic interactions across trophic levels. Philos Trans R Soc Lond B Biol Sci 365:2025–2034. doi:10.1098/rstb.2010.0037
van Emden HF (1991) The role of host plant resistance in insect pest mis-management. Bull Entomol Res 81:123–126
van Leur H, Raaijmakers CE, van Dam NM (2008) Reciprocal interactions between the cabbage root fly (Delia radicum) and two glucosinolate phenotypes of Barbarea vulgaris. Entomol Exp Appl 128:312–322. doi:10.1111/j.1570-7458.2008.00722.x
White TCR (1984) The abundance of invertebrate herbivores in relation to the availability of nitrogen in stressed food plants. Oecologia 63:90–105
White TCR (2009) Plant vigour versus plant stress: a false dichotomy. Oikos 118:807–808. doi:10.1111/j.1600-0706.2009.17495.x
Wilson PR (1990) A new instrument concept for nitrogen/protein analysis—a challenge to the Kjeldahl method. Aspects Appl Biol 25:443–446
Wyatt IJ, White PF (1977) Simple estimation of intrinsic increase rates for aphids and tetranychid mites. J Appl Ecol 14:757–766
Acknowledgments
We thank Rosemary Collier (University of Warwick) for supplying D. radicum. M. T. was financially supported by the Higher Education Commission of Pakistan. Four anonymous referees helped us to improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Stefan Scheu.
Rights and permissions
About this article
Cite this article
Tariq, M., Rossiter, J.T., Wright, D.J. et al. Drought alters interactions between root and foliar herbivores. Oecologia 172, 1095–1104 (2013). https://doi.org/10.1007/s00442-012-2572-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-012-2572-9