Soil Fungal Community Characteristics and Mycelial Production Across a Disturbance Gradient in Lowland Dipterocarp Rainforest in Borneo
- 1Lancaster Environment Centre, Lancaster University, Lancaster, United Kingdom
- 2UK Centre for Ecology & Hydrology, Lancaster, United Kingdom
- 3Department of Earth and Environmental Sciences, The University of Manchester, Manchester, United Kingdom
- 4Environmental and Rural Science, University of New England, Armidale, NSW, Australia
- 5Environmental Change Institute, School of Geography and the Environment, University of Oxford, Oxford, United Kingdom
- 6Department of Life Sciences, Imperial College London, Silwood Park, Ascot, United Kingdom
- 7UK Centre for Ecology & Hydrology, Wallingford, United Kingdom
- 8Forest Research Centre, Sabah Forestry Department, Sandakan, Malaysia
- 9UK Centre for Ecology & Hydrology, Environment Centre Wales, Bangor, United Kingdom
The rainforests of Southeast Asia are a global biodiversity hotspot under increasing pressure from human activity. Selective logging and forest conversion to oil palm plantation has major implications for biogeochemical cycling and carbon storage that are underpinned by plant-soil interactions. Soil fungi are key regulators of carbon and mineral nutrient flows between above- and below-ground organisms, yet understanding of fungal community-productivity relationships in hyper-diverse tropical forests is lacking. Recent studies suggest sensitivity of soil fungal communities to land-use change, although impacts on fungal productivity remain largely unresolved. To address this gap, we installed hyphal in-growth bags for 6 months in old-growth (OG) and selectively logged (SL) forest and oil palm plantation (OP) in Bornean lowland rainforest. Mycelial (actively foraging) fungal communities were characterized by ITS amplicon sequencing, and mycelial production estimated by measurement of fungal hyphae. Mycelial fungal community compositions were similar in OG and SL forest, whereas OP had significantly different communities of saprotrophic, mycorrhizal, and pathogenic fungi. In particular, total mycorrhizal and ectomycorrhizal fungal relative abundances, total mycorrhizal richness and mycelial production was reduced. However, due to restricted sampling replication in OP, effects associated with site could not be excluded. In forest plots (OG & SL), we further explored the broader drivers of mycelial fungal communities using tree community, structure and productivity data, and soil and environmental properties. Forest mycelial community dissimilarities were related to soil and vegetation characteristics, while mycelial production was broadly independent of these as well as fungal community attributes. An increase in arbuscular mycorrhizal relative abundance was also found with selective logging, which may have implications for carbon storage capacity in these forests, while an apparent retention of mycorrhizal mycelium in SL forest may act as a reservoir of inoculum that could aid forest restoration. Our results show that conversion of rainforest to oil palm plantation has significant consequences for fungal diversity-productivity relationships with implications for nutrient and carbon dynamics and restoration over large spatial scales.
Introduction
Tropical forests represent the most biodiverse terrestrial ecosystems on the planet (Myers et al., 2000) and provide a globally important carbon (C) sink (Pan et al., 2011). Increasingly rapid reductions in forest cover through land-use and land-conversion threatens the capacity of tropical forest to support biodiversity (Hansen et al., 2013; Powers and Jetz, 2019) and store C (Baccini et al., 2017). Significant forest degradation is occurring in Southeast Asia, with highest rates in Borneo (Bryan et al., 2013). Since the early 1970s, expansion of industrial oil palm plantation (OP) has resulted in the loss of >30% of forest cover (18.7 Mha of old-growth forest), while more than 70% of the remaining forest has been affected by selective logging for extraction of commercially valuable timber (Gaveau et al., 2014, 2016).
Ecosystem functions and biogeochemical cycles (e.g. C storage and nutrient dynamics) are underpinned by complex plant-soil interactions, mediated through reciprocal feedbacks between aboveground vegetation and soil microbial communities (Wardle et al., 2004; van der Heijden et al., 2008; Bever et al., 2010; van der Putten et al., 2013; Cortois et al., 2016). Changes in soil properties associated with forest disturbance have been shown to have significant and long-lasting effects on microbial communities and diversity across multiple biomes (Hartmann et al., 2013; Mcguire et al., 2015). However, despite the importance of plant-soil microbe interactions, understanding of relationships between above-below-ground communities alongside soil properties in tropical ecosystems remains limited (Barberán et al., 2015; Mueller et al., 2016). A small number of recent studies have highlighted the sensitivity of soil fungal communities to land-use change in Southeast Asia, including effects of selective logging and forest conversion to oil palm plantation on community structure and diversity (Kerfahi et al., 2014; Mcguire et al., 2015), although the direct impacts on functioning of fungal communities are unclear.
The mycelium produced by actively foraging soil fungi is a key component of C and mineral nutrient cycles in terrestrial ecosystems (Johnson et al., 2002; Finlay, 2008; Cairney, 2012). The extraradical mycelia of mycorrhizal fungi act as direct pathways for the reciprocal exchange of mineral nutrients from soils and photosynthetic C between plants and fungal symbionts. This mutualistic association benefits the host plant through increased surface area for the absorption of mineral nutrients from soil, in exchange for plant-derived photosynthates required for fungal growth and survival (Smith and Read, 2008; Itoo and Reshi, 2013; Chen et al., 2016). Mycorrhizal symbiosis is also known to improve plant resistance to disease and environmental perturbations including drought and exposure to harmful pollutants (Smith and Read, 2008). Plant productivity is enhanced through this mutually beneficial partnership (Lambers et al., 2008; Nasto et al., 2014; Wurzburger and Clemmensen, 2018), in turn leading to greater belowground C allocation by plants (Orwin et al., 2011) and C supply to the wider soil microbiome (Drigo et al., 2012; Nottingham et al., 2013). Recent research has evidenced nutrient transfer between plants of the same or even different species through common mycelial networks (CMNs) that physically connect two or more individuals, which can support interplant exchanges over long distances (Bever et al., 2010; Barto et al., 2012; Babikova et al., 2013; Gorzelak et al., 2015). While this process can influence plant survival and growth, it may also support establishment of young trees by providing existing infrastructure for seedlings to access (Nara, 2006; Gorzelak et al., 2015). While fungal hyphal structures themselves provide a C sink, they may also contribute to soil organic matter pools through mycelial turnover (Wallander et al., 2013) and protection of organic substrates through effects on soil aggregation (Wilson et al., 2009; Rillig et al., 2015). Although the precise role of mycelial production and activity in soil C dynamics is contentious and remains an area of active research (Zak et al., 2019), recent studies have highlighted links between mycorrhizal community structure and soil C storage. For example, dominance of ectomycorrhizal (EcM) vs. arbuscular mycorrhizal (AM) fungi may influence soil C accumulation potentially through competition with saprotrophic fungi for resources required for the decomposition of organic matter (Averill et al., 2014).
Mycelial production is regulated by a range of abiotic and biotic factors such as soil mineral nutrient availability (Hagerberg et al., 2003; Nilsson and Wallander, 2003; Potila et al., 2009; Ekblad et al., 2013), soil moisture (Majdi et al., 2008), climate (Bakker et al., 2015), phenological/seasonal changes in belowground C allocation (Ekblad et al., 2013), and direct grazing by soil mesofauna which may inhibit or stimulate mycelial production (Ek et al., 1994; Setäl et al., 1999). Plant and fungal community structures themselves are also critical for determining mycelial growth and turnover (Clemmensen et al., 2015), as shifts in vegetation and mycelial fungal communities related to forest management have been linked to patterns in mycelial production (Hagenbo et al., 2018). However, quantification of mycelial production rates has largely been confined to boreal and temperate biomes, mostly focussing on EcM-dominated systems in Scandinavia (Ekblad et al., 2013).
The lowland rainforests of Borneo are characterized by the high abundance and canopy dominance of tree species belonging to the obligate EcM-associating Dipterocarpaceae family (Whitmore, 1984; Taylor and Alexander, 2005; Brearley, 2012), with dipterocarp community assemblages potentially mediated by their mycorrhizal partners (Essene et al., 2017). Overall, the majority of tree species in these hyper-diverse forests also associate with AM fungi as in most tropical forests (Mcguire et al., 2008). Dipterocarps are directly targeted through selective logging due to their commercial value as timber (Appanah and Turnbull, 1998), making way for establishment of other tree species that are likely to be AM-forming (Mcguire et al., 2008). A recent study highlighted the effects of such tree community alterations on net primary productivity (NPP) allocation (Riutta et al., 2018), with shifts from canopy NPP to woody NPP fractions in selectively logged (SL) Bornean lowland dipterocarp rainforest. Corresponding shifts in tree functional traits have also been reported, indicating traits associated with C capture and growth to be more pronounced in SL forest compared to structural and persistence traits in old-growth (OG) forest (Both et al., 2019). These alterations to aboveground productivity patterns and plant functional traits along with modified soil properties due to logging may have strong implications for soil mycelial fungal community characteristics (i.e. the structure and diversity of actively foraging soil fungi), already indicated by shifts observed in bulk soil EcM fungal communities with SL (Mcguire et al., 2015), although these relationships and subsequent impacts on productivity to date have not been investigated. Establishment of OP involves the conversion of forest to expansive monoculture plantations of the AM-associating African species Elaeis guineensis (Phosri et al., 2010; Gaveau et al., 2014), and is associated with dramatic loss of forest-dwelling species, severely impacting on biodiversity (Fitzherbert et al., 2008; Sodhi et al., 2010; Wilcove et al., 2013). The soil microbiome is no exception, with previous studies documenting pronounced differences in both bacterial and fungal communities in OP relative to OG and SL forest, which may be relatively more similar (Lee-Cruz et al., 2013; Kerfahi et al., 2014; Mcguire et al., 2015). In particular, OP can greatly reduce richness and relative abundance of EcM fungi, which may be almost entirely absent from this land-use (Kerfahi et al., 2014; Mcguire et al., 2015). These alterations may have drastic consequences for mycelial productivity and related ecosystem functions, but impacts remain as yet unresolved.
The overarching aim of this study was to evaluate the impact of selective logging and forest conversion to oil palm plantation on mycelial fungal community characteristics and productivity, and explore the role of soil and vegetation properties as drivers of mycelial fungal community composition and productivity in tropical lowland dipterocarp rainforest. We constructed our experiment to address the following hypotheses:
H1. Mycelial fungal community attributes will differ across land-use types, with more distinct communities in OP relative to OG and SL forest (Mcguire et al., 2015). EcM relative abundance is expected to be highest in OG forest, followed by SL forest and OP (Kerfahi et al., 2014). This will be reflected in rates of mycelial production which will decline with disturbance intensity, along with removal of EcM-associating trees. Differences observed will correspond to alterations in soil and environmental properties, tree community composition and functional characteristics between land-use types resulting from disturbance.
H2. Across forest land-uses, mycelial fungal community attributes and production will be explained by a combination of soil and environmental properties, tree community composition and functional characteristics (see above).
H3. Across forest land-uses, shifts in mycelial fungal community attributes (particularly mycorrhizal) will correlate with rates of mycelial production (Clemmensen et al., 2015; Hagenbo et al., 2018).
Methods
Study Sites
This study was carried out in the Malaysian state of Sabah, northern Borneo. This region is characterized by a moist tropical climate, with average daily temperature of 27°C and annual precipitation of 2,600–2,700 mm (Walsh and Newbery, 1999; Kumagai and Porporato, 2012). Its central Southeast Asian location is considered aseasonal, with no distinct wet or dry season (Richards, 1996; Dykes, 1997; Itioka and Yamauti, 2004), although experiences irregular inter-annual dry periods which may average a total of ~1.4 months per year (Walsh and Newbery, 1999; Kumagai et al., 2005). Sampling was conducted in a total of nine 1 ha plots across OG and SL lowland dipterocarp rainforest and OP (see Supplementary Information 1 for map of sampling locations). Four plots were distributed across OG forest: two plots were situated in the Danum Valley Conservation Area (DVCA) (4.951°, 117.796° and 4.953°, 117.793°), and two in the Maliau Basin Conservation Area (MBCA) (4.747°, 116.970°, and 4.754°, 116.950°), coded as DAN-04, DAN-05, MLA-01 and MLA-02, respectively in the ForestPlots database (www.forestplots.net). These reserves have undergone little or no anthropogenic disturbance with legal protection from logging granted in 1976 and 1981, respectively (Marsh and Greer, 1992; Hazebroek et al., 2004). Four plots were located in SL forest in the Kalabakan Forest Reserve (SAF-01: 4.732°, 117.619°; SAF-02: 4.739°, 117.617°; SAF-03: 4.691°, 117.588° and SAF-04: 4.765°, 117.700°) and one in OP (4.641°, 117.452°; Benta Wawasan Sdn. Bdh.), in established research sites within the large-scale forest fragmentation study Stability of Altered Forest Ecosystems (SAFE) Project (Ewers et al., 2011). Two SL plots had been selectively logged twice and two four times, with forests undergoing timber extraction first during the mid-1970s (~113 m3 ha−1 timber removed), followed by up to three more times between 1990 and 2008 (~37–66 m3 ha−1 cumulative timber removed) (Fisher et al., 2011; Riutta et al., 2018). The OP plot was located in a stand aged ~7 years at the time of sampling, where fertilizer applications were roughly two applications of 3–4 kg bags palm−1 year−1 (comprising a mixture of diammonium phosphate, potassium chloride, ammonium sulfate, magnesium sulfate, and borax pentahydrate) prior to this study (J. Drewer, personal communication). All plots in OG and SL forest were previously established as part of the Global Ecosystem Monitoring (GEM) network (http://gem.tropicalforests.ox.ac.uk/) for the long-term evaluation of forest carbon cycling and productivity since 2011 (Malhi et al., 2015; Riutta et al., 2018), and have recently been characterized for tree community assemblage, plant traits, soil microbial communities and soil physicochemical properties (Elias et al., 2018; Both et al., 2019).
Sampling Design
Three 20 × 20 m subplots were randomly chosen per 1 ha plot for assessing mycelial community attributes and productivity. Ten hyphal in-growth bags were installed per subplot at a randomly chosen location. Bags were buried at 50 cm intervals along two parallel transects (5 bags per transect) spaced 1 m apart to ensure adequate sample recovery and account for spatial variability in fungal community composition and mycelial production.
Hyphal In-Growth Bags
Hyphal in-growth bags were constructed from two 5 cm × 5 cm squares of fine pore-size nylon mesh (Plastok, UK) sealed with a soldering iron. A mesh size of 41 μm was chosen to allow access to fungal hyphae, but prevent ingress of plant roots (Fisher et al., 2013; Wallander et al., 2013). Bags were filled with 25 g oven-dried, heat-sterilized quartz sand (150°C for 24 h). In-growth bags were installed between April-May 2016 by burying vertically at the soil surface covering a depth of ~0–5 cm. This was chosen to include the organic layer and interface between organic and mineral horizons to maximize fungal in-growth (Lindahl et al., 2007; Wallander et al., 2013). Canopy litterfall tends to be relatively stable throughout the year in these forests, reflecting little inter-annual climatic variation (Kumagai and Porporato, 2012). Therefore, in-growth bags were harvested after a period of 6 months to allow fungal colonization, rather than the duration of a growing season which is commonly used in temperate or boreal systems (Wallander et al., 2013). Bags were frozen in a field laboratory upon collection, and transported on ice to Lancaster University, UK where they were stored at −20°C prior to analysis. Bags were examined and discarded where there was clear damage or root ingress. Due to low recovery of undamaged bags, three in-growth bags were used per subplot to account for local variation in mycelial colonization (with the exception of five composite samples: one bulked from two in-growth bags, and four from one bag due to high damage or loss rates in some subplots). Bags were opened in the laboratory and sand was carefully bulked per subplot and hand-mixed, providing 27 composite samples (three per 1 ha plot) for quantification of mycelial abundance and molecular analysis of fungal communities. Only one 1 ha plot was established in the OP site, due to logistical challenges and as spatial variation in this land-use type was expected to be low (as suggested by low fungal beta diversity and variation in EcM relative abundances in OP relative to OG and SL in Borneo; Kerfahi et al., 2014). As such the three composite OP samples were treated as independent replicates, each representing an individual plot-level sample for all analyses.
Hyphal Extraction and Estimation of Mycelial Productivity
Hyphae were extracted from sand using an adapted floatation method (Bakker et al., 2015). Five-gram subsamples were transferred into 50 ml sample tubes, then filled up to 30 ml with 4 M KCl solution. This extractant was used to ensure floatation of all hyphae and avoid clumping and sinking of fungal filaments, which may be hydrophilic or hydrophobic (Ekblad et al., 2013), preventing adequate separation from sand particles (as found in prior laboratory tests using distilled water). Tubes were vortexed at full speed for 1 min, left to stand for 30 s to allow hyphal material to reach the surface of the solution, and 25 ml was decanted into a clean sample tube, avoiding the transfer of sand particles. This process was repeated once more on the same sand subsample. The extract was transferred into a sample pot and made up to 100 ml by rinsing the sample tube twice with 25 ml distilled water. Hyphal material was prepared for measurement using the membrane filtration technique (Hanssen et al., 1974). The extract was evenly mixed using a sample mixer at 700 rev min−1, and 10 ml aliquots were transferred in two steps using a 5 ml pipette during mixing into a 15 ml glass filtration tube mounted on a nitrocellulose filter membrane (Merck Millipore, USA; 1.2 μm pore-size, 25 mm diameter). The suspension was filtered with a vacuum pump, and hyphae were stained in the tube using Lactophenol cotton blue under a fume hood for 20 min. The filter membrane was then rinsed with distilled water until filtrate ran clear, and left to air dry overnight. Filter membranes were mounted onto microscope slides with immersion oil for transparency and sealed under a coverslip.
Hyphae on filter membranes were photographed at high resolution (4,080 × 3,072 pixels) using a microscope-mounted camera at 100x magnification (Olympus BX51, Olympus DP71), allowing identification of hyphal structures with diameters ≥ 1 μm. Photographs were used for hyphal measurement to maximize membrane coverage and capture of within-sample variation not possible through direct microscope observation at higher magnification. Twenty-five photographs (each covering an area of ~1.53 mm2) were taken per membrane along four crossing transects positioned at 45° to each other, as described by Boddington et al. (1999). Hyphal length was estimated from photographs using the gridline intersect method (Tennant, 1975). A regular grid of grid size 50 × 50 μm was digitally placed on top of each photograph using ImageJ (Schneider et al., 2012; Shen et al., 2016). Image contrast and brightness were altered as necessary to improve visualization of hyphae. All intersections between hyphae and gridlines were counted, and total hyphal length was calculated using Tennant's formula. The total hyphal length in each subsample was estimated using hyphal length per area in photographs and area of membrane used for filtration. 10 g of fresh sand from each composite sample was oven-dried at 105°C to constant weight to calculate moisture content for standardization of hyphal length estimations. Hyphal length was then calculated in mm g−1 dry sand for statistical analysis of mycelial production.
Soil, Environmental and Vegetation Characteristics
Soil physicochemical data for corresponding subplots were obtained from an existing dataset (Elias et al., 2018). Briefly, five soil samples were collected within each subplot (3 cm diameter gouge auger) March-April 2015. Organic soil layer depth was measured before separation from underlying mineral soil. Organic layer soil samples were bulked per subplot and analyzed for organic layer pH, total C, total nitrogen (N), total phosphorus (P), inorganic P and texture (% sand, silt and clay). pH in water was measured on fresh soils using a pH meter (1:2.5 soil to deionised water) after shaking overnight at 100 rev m−1 on an orbital shaker and standing for 30 min (Landon, 1984). The remaining soils were air-dried at 40°C to constant weight and passed through a 2 mm sieve. Subsamples for total C and N analysis were dried at 65°C for 48 h and milled to a fine powder with a pestle and mortar. Total soil C and N contents were determined by dry combustion at 900°C using an Elementar Vario Max CN analyser (Elementar Analysensysteme, Hanau, Germany). Samples were digested using sulphuric acid-hydrogen peroxide (Allen, 1989) for soil total P. Inorganic P was extracted using a Bray No. 1 extractant (Bray and Kurtz, 1945). P contents of extracts and digests were determined using the molybdenum-blue method (Anderson and Ingram, 1993), read at 880 nm on a spectrophotometer (HITACHI-UV-VIS, Japan). Soil texture was measured by the pipette method (Miller and Miller, 1987). Soil bulk density was determined from one additional sample taken per subplot using a 7.5 cm diameter volumetric ring, dried at 105°C for 24 hours after removal of roots and stones (Emmett et al., 2008). Soil moisture content (top 12 cm), temperature (10 cm depth) and air temperature (20 cm above soil surface) values for corresponding subplots were accessed from datasets of continuous sampling of GEM plots, as described in Marthews et al. (2014). Slope measurements were taken upon in-growth bag harvesting using a clinometer at each subplot corner and center, and values were averaged at the subplot level. Altitude was recording using a GPS in each subplot center. Forest structural characteristics [stem density, basal area, and mean and maximum diameter of stems at breast height (DBH) ≥ 10 cm, pioneer tree proportion of basal area, and Leaf Area Index (LAI)] and tree productivity metrics [canopy net primary production (NPP), woody NPP, root NPP, total NPP] for corresponding subplots were also accessed from GEM plot datasets, using temporally-averaged data collected between 2011 and 2016 (Marthews et al., 2014; Riutta et al., 2018). Tree taxonomic community datasets were constructed at the 1 ha plot level from a previous survey undertaken July-December 2015, where all individual trees DBH ≥ 10 cm within three 20 × 20 m subplots were taxonomically identified (Both et al., 2019). Dipterocarp basal area was derived from tree community data at the plot level.
Molecular Analysis of Mycelial Fungal Communities and Data Pre-Processing
DNA was extracted from 0.2 g sand from in-growth bags using the PowerSoil® DNA Isolation Kit and protocol (MoBio Laboratories). Amplicon libraries were constructed according to a dual indexing strategy with each primer consisting of the appropriate Illumina adapter, 8-nt index sequence, a 10-nt pad sequence, a 2-nt linker and the amplicon specific primer (Kozich et al., 2013). Fungi were targeted by amplifying the ITS2 region using primers GTGARTCATCGAATCTTTG and TCCTCCGCTTATTGATATGC (Ihrmark et al., 2012). Although the capability of detecting AM fungi using ITS primers is debated (Hart et al., 2015), recent studies have shown that patterns in diversity and community composition can be adequately identified within sample types such as soil (Berruti et al., 2017; Lekberg et al., 2018). Amplicons were generated using a high fidelity DNA polymerase (Q5 Taq, New England Biolabs). After an initial denaturation at 95°C for 2 min, PCR conditions were as follows: Denaturation at 95°C for 15 s; annealing at 52°C; annealing times were 30 s with extension at 72°C for 30 s; cycle numbers were 25; a final extension of 10 min at 72°C was included. Amplicon sizes were determined using an Agilent 2200 TapeStation system, samples were normalized using SequalPrep Normalization Plate Kit (Thermo Fisher Scientific) and pooled. The pooled library was quantified using a Qubit dsDNA HS kit (Thermo Fisher Scientific) prior to sequencing with an Illumina MiSeq using V3 600 cycle reagents at a concentration of 8 pM with a 5% PhiX Illumina control library. The sequencing run produced in excess of 18 million reads passing filter. Sequences were processed in R (R Core Team, 2013) using the DADA2 package to quality filter, merge, de-noise and assign taxonomies (Callahan et al., 2016). Sequence reads were trimmed to 225 and 160 bases, forward and reverse, respectively. Filtering settings were maximum number of Ns (maxN) = 0, maximum number of expected errors (maxEE) = 1. Sequences were dereplicated and the DADA2 core sequence variant inference algorithm applied. mergePairs and removeBimeraDenovo functions were used at default settings to merge forward and reverse reads and remove chimeric sequences. The amplicon sequence variants (ASVs) were subject to taxonomic assignment using assignTaxonomy and the training database UNITE version 7.2 (UNITE Community, 2017).
Fungal functional guild classifications were assigned to ASVs using the FUNGuild annotation tool (Nguyen et al., 2016). Only ASVs with unambiguous (non-multiple) classifications of “probable” or “highly-probable” confidence rankings were considered for analysis. These were used for calculating relative abundances of fungal guilds and sub-setting saprotrophic, mycorrhizal and pathogenic fungal datasets for assessment of diversity and community dissimilarity (EcM and AM fungal diversity and community dissimilarities could not be analyzed separately due to absence of read counts in a number of samples). Sequencing data were pre-processed (steps described below) and alpha diversity indices (ASV richness, Shannon index) and fungal guild relative abundances calculated in R version 3.5.1 (R Core Team, 2013) using the phyloseq package (Mcmurdie and Holmes, 2013). Only ASVs assigned to the kingdom of Fungi were retained for downstream analysis (99.34% of total reads), and all singleton ASVs were removed. Sub-setting by fungal guilds was conducted on the full unrarefied dataset to maximize the number of ASV reads available for analysis of functional groups. Sample sequencing depth was normalized for each group by rarefying to the minimum read counts of 4881, 151, 75, and 67 per sample for overall, saprotrophic, mycorrhizal and pathogenic fungal groups, respectively. Analyses of diversity metrics and community dissimilarities were repeated using unrarefied and whole-sample only rarefied datasets (before sub-setting fungal groups), which showed broadly consistent results.
Statistical Analyses
All statistical analyses were conducted in R version 3.5.1 (R Core Team, 2013) and significance was considered at the p ≤ 0.05 level. For univariate analyses, linear mixed effects regression models (LMMs) were constructed in the lme4 R package (Bates et al., 2015). This included testing (H1) the effect of land-use type on relative abundances of mycelial fungal guilds and alpha diversity, mycelial production and soil, environmental and vegetation characteristics, and (H2 & H3) relationships between these variables across OG and SL plots. Post-hoc pairwise comparisons were conducted with the emmeans R package (Lenth et al., 2019) with Bonferroni correction to identify statistically different variable means between OG, SL and OP land-use types. To control for potential within-plot pseudoreplication, plot ID was included as a random intercept term. Significance was evaluated using the Satterthwaite degrees of freedom approximation (Luke, 2017). Normality of model residuals were evaluated using Shapiro-Wilk tests, and variables were log- and exponentially-transformed where necessary to improve model fit. Kruskal-Wallis tests were conducted when residual normality could not be satisfactorily achieved using data averaged at the plot level.
Mycelial fungal community compositions across all land-use types were visualized with PCoA using Bray-Curtis dissimilarities via the phyloseq, vegan (Oksanen et al., 2019) and ggplot2 (Wickham, 2016) packages. To test differences in fungal community compositions between OG, SL and OP (H1), Bray-Curtis community dissimilarities were calculated from data averaged at the plot level (n = 11) using the merge_samples function in phyloseq. Differences between land-use types were tested with PERMANOVA using the adonis vegan function, and statistically different groups were identified using the pairwise.adonis function in the pairwiseAdonis R package (Martinez Arbizu, 2019) controlling for the False Discovery Rate (FDR). All permutational tests were run with 9,999 permutations with the exception of pairwise multiple comparisons, where full enumeration was used. Indicator analyses were conducted to identify specific fungal taxa associated with different land-use types using the labsdv R package (Dufrêne and Legendre, 1997; Roberts, 2016). Differences in tree community composition between OG and SL forest (H1) were tested with PERMANOVA using Bray-Curtis community dissimilarities calculated from plot-level community data. Hellinger-transformation was applied to all community data prior to multivariate analyses (Legendre and Borcard, 2018) to control for the effect of rare taxa. Homogeneity of multivariate dispersion across land-use types (an assumption of PERMANOVA) for fungal and tree community dissimilarities was evaluated using the betadisper vegan function for overall and pairwise tests (FDR-corrected).
Relationships between mycelial fungal community compositions and soil, environmental and vegetation characteristics (H2) across forest (OG and SL) plots were evaluated using distance-based redundancy analysis (db-RDA). Tree community composition was included in analysis as represented by the first three PCoA axes, explaining most of the variation (60.39% cumulative eigenvalues). Prior to analysis, highly correlated variables within 1) soil and environmental and 2) vegetation groups were identified with correlograms using the corrplot R package (Wei and Simko, 2017). Variables correlated with Pearson's r ≥ ~ 0.7 were removed. Soil and environmental variables were treated as one group as soil characteristics were considered to be of primary ecological importance, while driven by environmental factors. The best predictors of fungal Bray-Curtis dissimilarities were identified through forward-selection using the criteria of adjusted R2 and significance level of p < 0.05 (Blanchet et al., 2008) with the ordiR2step vegan function. Here, Bray-Curtis dissimilarities were calculated at the subplot level and permutational tests were restricted by plot ID to control for the nested sampling design using the permute R package (Simpson et al., 2019). Variation in fungal community dissimilarities was partitioned by soil and environmental and vegetation components using the varpart function, and significance of components and individual predictors was tested with partial db-RDA in vegan. Relationships between mycelial fungal and vegetation communities (H2) were tested using plot-level Bray-Curtis dissimilarities with Mantel tests (Spearman's rank correlation) in vegan. Relationships between mycelial fungal community compositions and mycelial production (H3) were tested with PERMANOVA using Bray-Curtis dissimilarities and the same permutational scheme described above.
Results
Overall, 3,565 fungal ASVs from 10 phyla (Figure 1) and 374 genera were detected across all samples of OG forest, SL forest and OP. Of the ASVs that were assigned fungal functional guilds, the majority of reads comprised saprotrophic (39.9%), followed by mycorrhizal [27.0%: 83.1% of which EcM; 16.7% AM; 0.2% ericoid mycorrhizal (ErM)], pathogenic (21.0%: 51.4% of which animal pathogenic; 48.6% plant pathogenic), parasitic (7.1%), endophytic (2.2%), lichenised (2.0%) and epiphytic (0.7%) fungi.
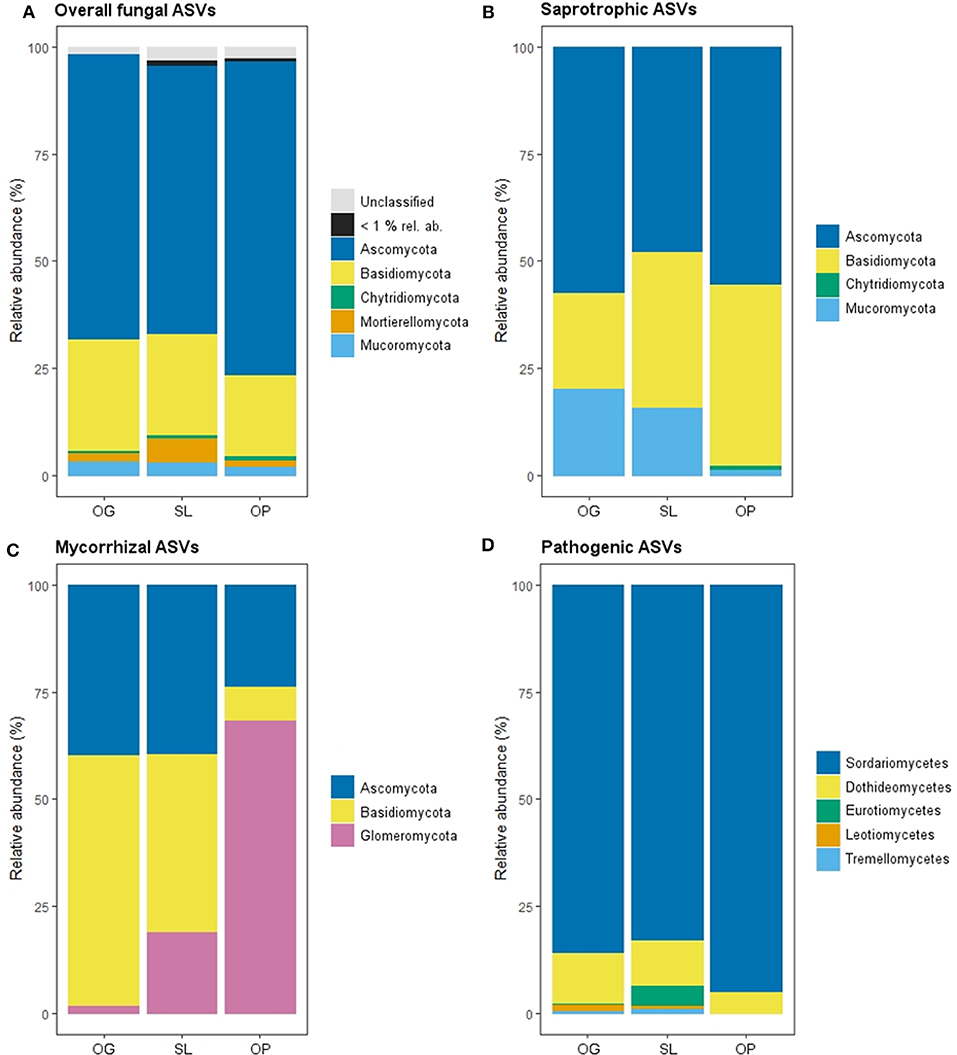
Figure 1. Relative abundance of mycelial fungal phyla as a percentage of total Amplicon Sequence Variants (ASVs) in old-growth (OG) and selectively logged (SL) lowland dipterocarp rainforest and oil palm plantation (OP) in Sabah, Borneo, for (A) overall, (B) saprotrophic, (C) mycorrhizal fungal groups. Phyla with <1% relative abundance across all forest land-use types are represented as one group for overall fungi. (D) Relative abundances of classes of mycelial pathogenic fungi. Phyla are not shown for this fungal group as reads were dominated by one phylum (Ascomycota: 98.76% in OG, 100% in SL and 99.38 in OP) with the remaining reads comprising taxa of the Basidiomycota.
H1 - Impact of Land-use Type on Mycelial Fungal Community Attributes, Mycelial Productivity and Soil, Environmental, and Vegetation Characteristics
Land-use type significantly affected community dissimilarities for overall, saprotrophic, mycorrhizal and pathogenic fungal groups (Figures 2A–D; see Table 1 for summary of statistics). Pairwise comparisons identified significant differences between OP and forest land-uses for overall and pathogenic fungal community dissimilarities. No significant differences were found between land-use types for saprotrophic or mycorrhizal community dissimilarity in pairwise comparisons (p > 0.05). However, further tests using only OG and SL plots showed no significant effect of land-use type on saprotrophic or mycorrhizal community dissimilarities (PERMANOVA: p = 0.223; p = 0.115, respectively), indicating OP to be the driver of the significant effect found across the full disturbance gradient for these fungal groups. Community dissimilarity dispersions were homogenous between all land-use types for all fungal groups (betadisper: p > 0.05). No significant differences were found in community dissimilarities or alpha diversity metrics (ASV richness and Shannon index) between OG and SL for any fungal group (p > 0.05), despite significant differences in soil, environmental and vegetation properties between forest land-uses (means by land-use type summarized in Table 2; test statistics for significant differences are reported in Supplementary Information 2). However, relative abundance of AM fungi was significantly higher in SL relative to OG (Figure 3) and ErM fungal relative abundance was higher in SL relative to all other land-use types (Figure 3) (see Table 3 for means of all mycelial fungal community attributes by land-use type; test statistics for significant differences are reported in Table 4). Overall pathogenic fungal relative abundance was marginally significantly higher in SL compared to OG when tested without OP samples (F = 4.25, R2 = 0.16, p = 0.051). The vast majority of mycorrhizal reads were attributed to EcM fungi in forest plots (89.6%: 98.31% in OG; 80.90% in SL), but were mostly AM fungi in OP (69.4% AM; 30.6% EcM). Total mycorrhizal and EcM fungal relative abundances were significantly lower in OP compared to forest plots (Figure 3). Mean parasitic fungal relative abundance was an order of magnitude higher in OP relative to OG and SL, although no significant differences between land-use types were found due to the considerable variation in OP samples (post-hoc tests: p > 0.05). For alpha diversity metrics, only mycorrhizal fungal ASV richness was significantly affected by land-use type (F = 5.80, R2 = 0.33, p = 0.025; Figure 4A), with lower values in OP compared to forest plots (OG—OP: p = 0.028; SL—OP: p = 0.018). Alpha diversity indices (ASV richness, Shannon index) did not differ between OG and SL for any fungal group (post-hoc tests: p > 0.05). Indicator analysis identified certain fungal taxa of the families Aspergillaceae, Sporocadaceae, and Nectriaceae to be most indicative of OG forest, while those of Ophiocordycipitaceae were characteristic of SL forest and Nectriaceae, Aspergillaceae and Ophiocordycipitaceae characteristic of OP (Supplementary Information 3).
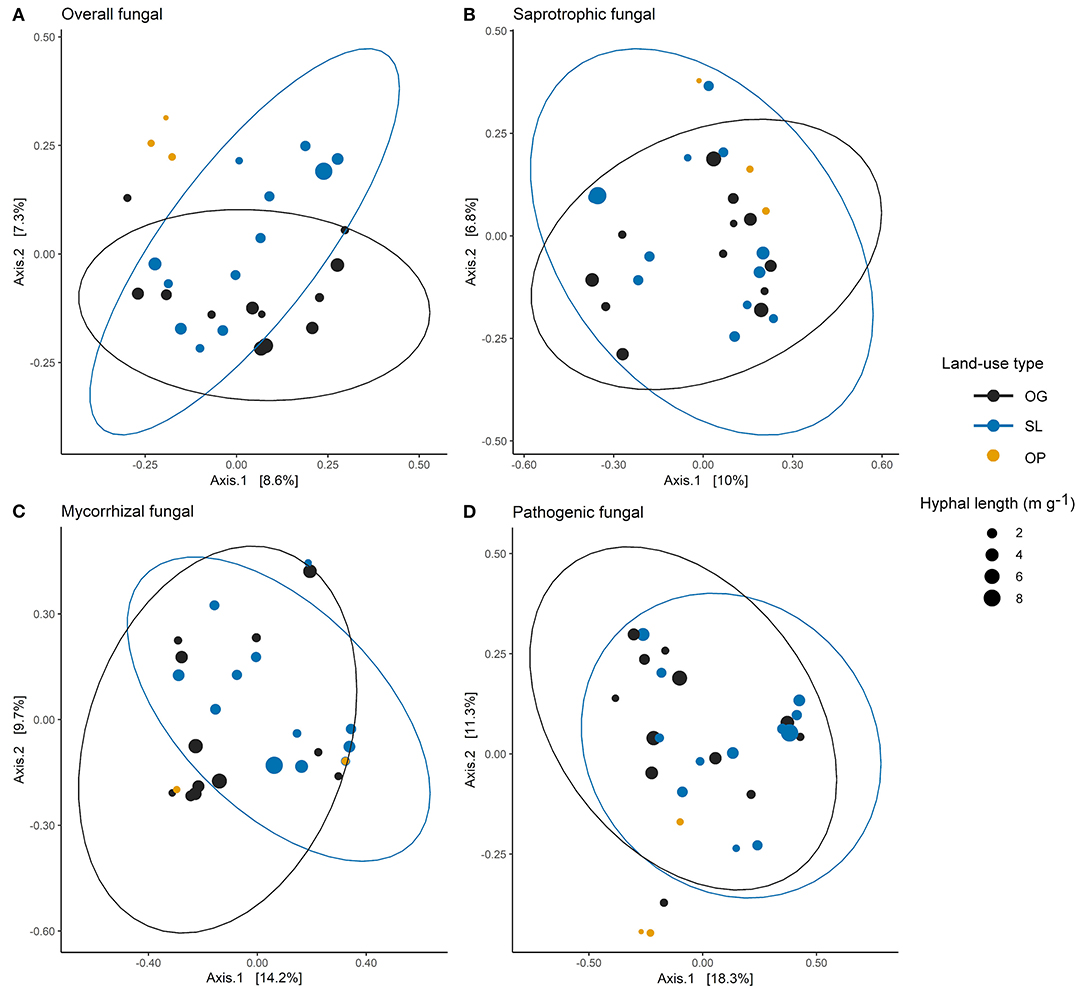
Figure 2. Principle coordinates analysis (PCoA) ordination of (A) overall, (B) saprotrophic, (C) mycorrhizal and (D) pathogenic mycelial fungal community Bray-Curtis dissimilarities across old-growth (OG) and selectively logged (SL) lowland dipterocarp rainforest and oil palm plantation (OP) in Sabah, Borneo. Points are scaled by hyphal length indicating mycelial production. Ellipses represent t-distribution confidences for OG selectively SL forest. Ellipses are not included for OP samples due to number of samples (n = 3). For mycorrhizal fungal community dissimilarities (C), two OP points are indistinguishable (hyphal length values 0.24 and 0.84 m g−1 respectively) due to sharing the same PCoA scores for Axes 1 and 2 (coordinates: 0.32, −0.12).
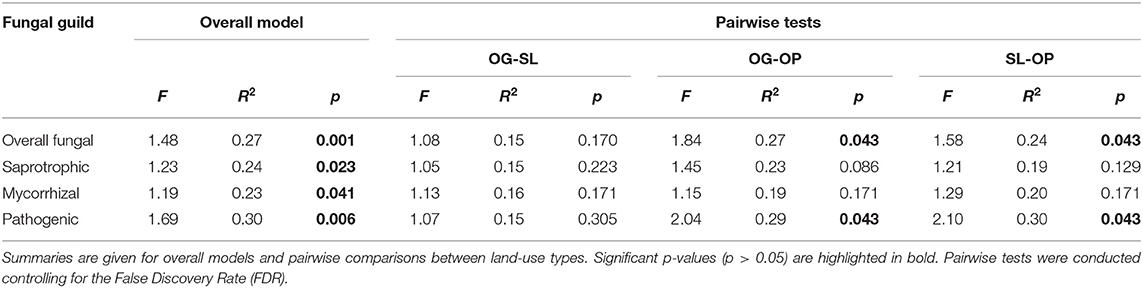
Table 1. PERMANOVA test statistics for differences in fungal guild community dissimilarities by land-use types of old-growth (OG) and selectively logged (SL) lowland dipterocarp rainforest and oil palm plantation (OP) in Sabah, Borneo.
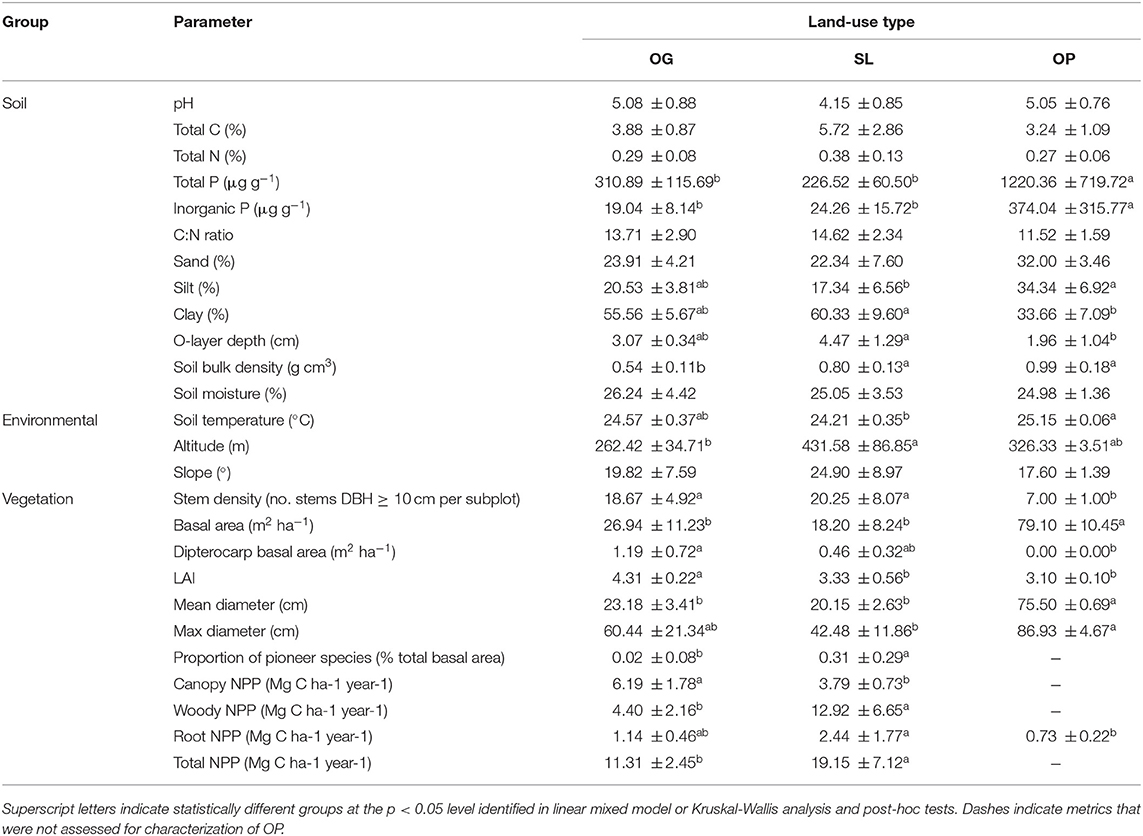
Table 2. Means (±1 SD) of soil, environmental, and vegetation characteristics by land-use type for old-growth (OG) and selectively logged (SL) lowland dipterocarp rainforest and oil palm plantation (OP) in Sabah, Borneo.
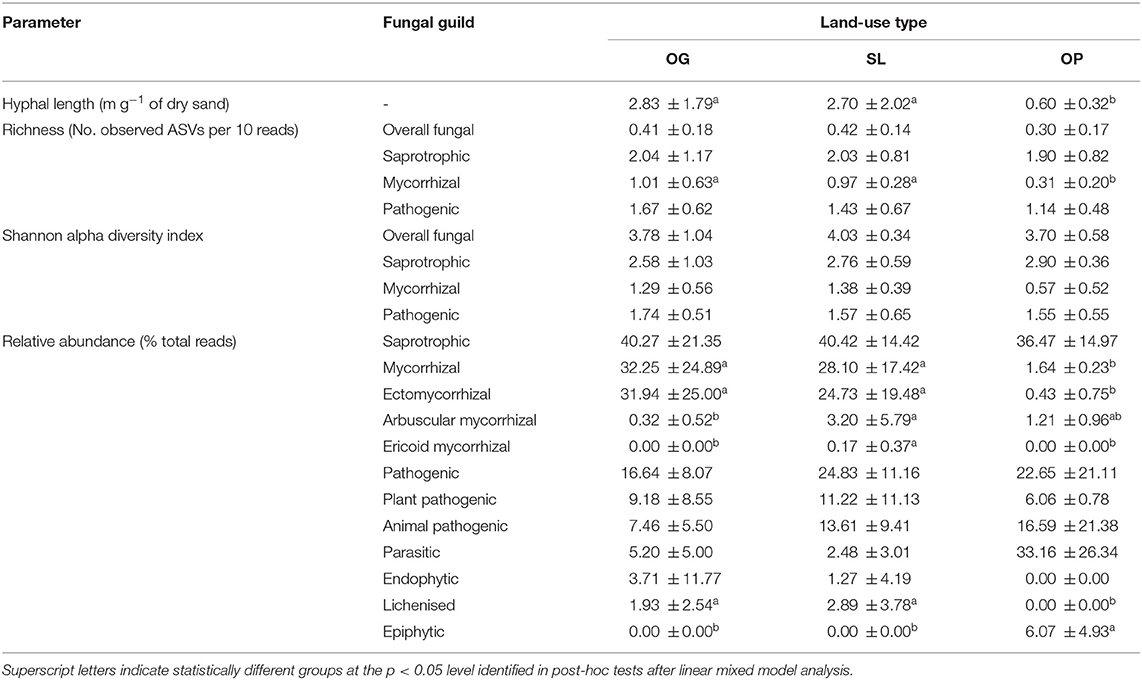
Table 3. Mean (± 1 SD) mycelial production (hyphal length) and fungal guild community attributes (Amplicon Sequence Variant (ASV) richness, Shannon alpha diversity and relative abundances) by land-use type for old-growth (OG) and selectively logged (SL) lowland dipterocarp rainforest and oil palm plantation (OP) in Sabah, Borneo.
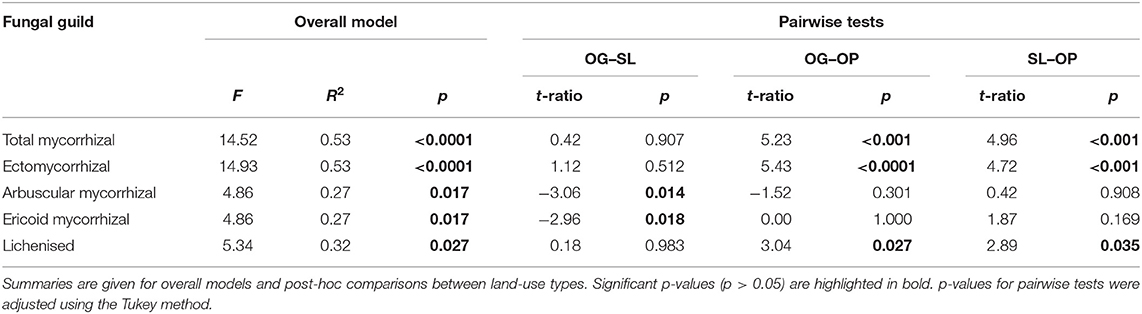
Table 4. Linear mixed model (LMM) test statistics for significant differences in fungal guild relative abundances by land-use types of old-growth (OG) and selectively logged (SL) lowland dipterocarp rainforest and oil palm plantation (OP) in Sabah, Borneo.
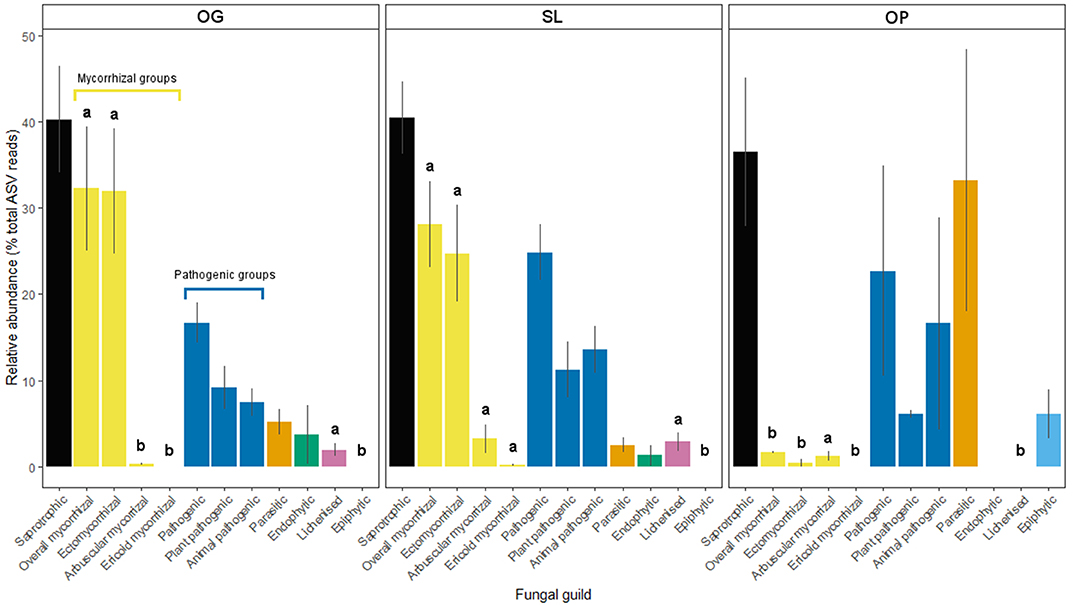
Figure 3. Relative abundances of fungal guilds for old-growth (OG) and selectively logged (SL) lowland dipterocarp rainforest and oil palm plantation (OP) in Sabah, Borneo. Error bars represent standard errors. Lower case letters indicate statistically different or similar groups across all three land-use types identified by post-hoc tests after linear mixed modeling or Kruskal-Wallis analysis (p < 0.05). Mycorrhizal and pathogenic guilds have been further divided into subtypes as indicated.
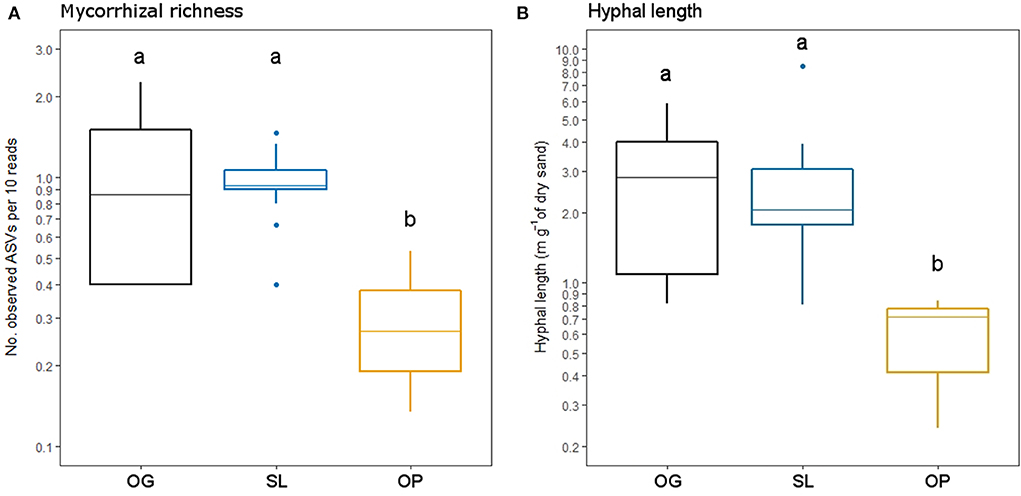
Figure 4. (A) Mycorrhizal Amplicon Sequence Variant (ASV) richness and (B) mycelial productivity (hyphal length) in old-growth and selectively logged lowland dipterocarp rainforest and oil palm plantation in Sabah, Borneo. Lower case letters indicate statistically different or similar groups across all three land-use types identified by post-hoc tests (p < 0.05). Values are shown on a log scale to represent differences corresponding to statistical tests.
Hyphal length values ranged from 0.24 to 8.50 m g−1 of dry sand across the disturbance gradient, and were significantly affected by land-use type (F = 6.50, R2 = 0.33, p = 0.006; Table 3; Figure 4B). Hyphal length values were significantly lower in OP relative to forest land-use types (OP–OG: p = 0.007; OP—SL: p = 0.008) but did not significantly differ between OG and SL (p = 0.999).
Relative to OG, significantly higher values were associated with SL for soil bulk density, altitude and proportion of pioneer species, and lower values for LAI and canopy, woody and total NPP (Table 2; Supplementary Information 2). Root NPP, soil clay content and organic layer depth in OP were significantly lower relative to SL, while soil silt content was significantly higher. OP soil bulk density was significantly higher relative to OG. Total and inorganic soil P were an order of magnitude higher in OP relative to both OG and SL. While stem density was significantly lower in OP relative to both forest types, basal area and mean stem diameter were significantly higher in OP. Maximum stem diameter was also higher in OP relative to SL, however all belonging to the same species of oil palm. Plot-level tree community dissimilarity significantly differed between OG and SL (PERMANOVA: F = 1.43, R2 = 0.19, p = 0.028; betadisper: p > 0.05).
H2 and H3 - Relationships Between Forest Mycelial Fungal Community Attributes, Soil, Environmental and Vegetation Characteristics and Mycelial Production
Overall, saprotrophic, mycorrhizal and pathogenic fungal community dissimilarities were significantly related to soil and vegetation characteristics, although overall variance explained was low for each group (see Table 5 for summary of statistics). The best predictors selected for overall fungal community dissimilarity were soil pH and LAI. Of these, soil pH was negatively related to relative abundance of animal pathogenic fungi (F = 8.63, R2 = 0.27, p = 0.008), while LAI was negatively related to relative abundance of AM fungi (F = 8.04, R2 = 0.28, p = 0.015). The only predictor selected for saprotrophic community dissimilarity was soil inorganic P, while the best predictors for mycorrhizal fungal community dissimilarity were pH and dipterocarp basal area. Soil pH, tree community PCoA axis 2, tree basal area and soil inorganic P were all identified as predictors of pathogenic fungal community dissimilarity. Plot- level tree community dissimilarity was significantly related to plot-level community dissimilarity of mycorrhizal fungi (r = 0.55, p = 0.015; Figure 5), but no other fungal group (p > 0.05). Dipterocarp and mycorrhizal fungal community dissimilarities were not significantly related at the plot-level (p > 0.05). Additional tests showed no significant relationship between either EcM or AM fungal relative abundance and soil C content (p > 0.05).
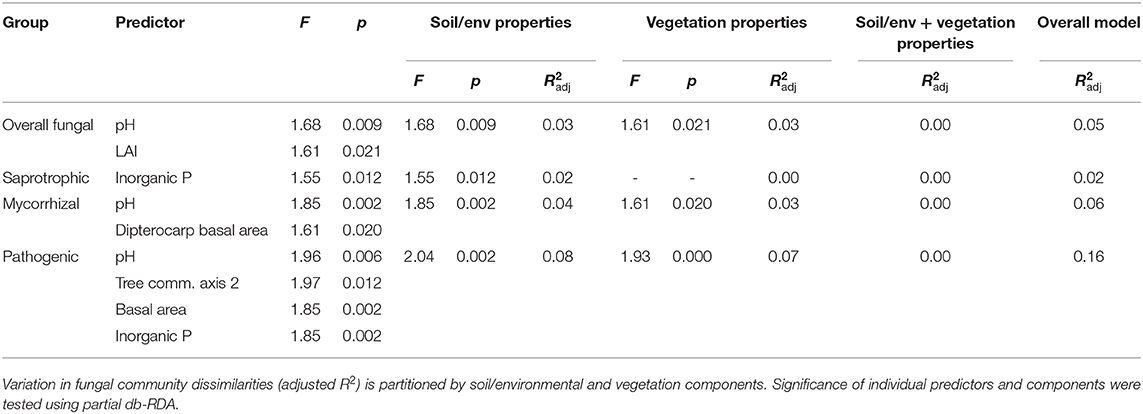
Table 5. Best soil, environmental and vegetation characteristics as predictors of fungal group dissimilarities across old-growth and selectively logged lowland dipterocarp rainforest in Sabah, Borneo, identified by forward selection (adjusted R2 and p > 0.05) in distance-based redundancy analysis (db-RDA).
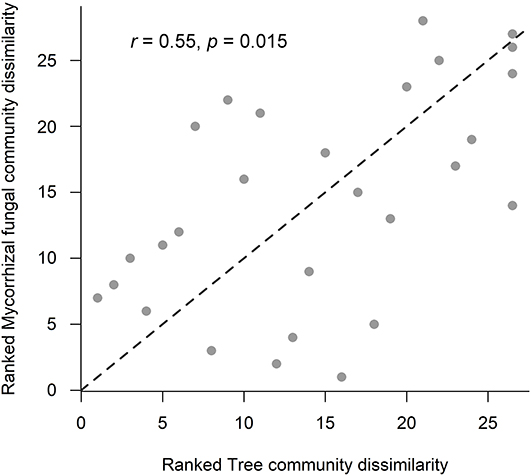
Figure 5. Relationship between ranked tree and mycorrhizal community Bray-Curtis dissimilarities across old-growth and selectively logged lowland dipterocarp rainforest in Sabah, Borneo. Statistics were provided by Mantel test with Spearman's rank correlation. The r-value represents the Mantel coefficient, and significance level was calculated using 9,999 permutations.
Hyphal length was not significantly associated to any mycelial fungal community attributes (H3), although a negative relationship with EcM fungal relative abundance was marginally significant (F = 3.57, R2 = 0.13, p = 0.072). There was no significant link between hyphal length and any of the vegetation properties measured (H2), including tree productivity metrics, but was significantly positively related to soil inorganic P (F = 5.25, R2 = 0.19, p = 0.032; Figure 6). However, the association between hyphal length and soil inorganic P was primarily driven by one sampling point in SL forest with high values in both parameters (Figure 6), and was found to be non-significant when this data point was excluded from analysis (F = 0.83, R2 = 0.04, p = 0.371).
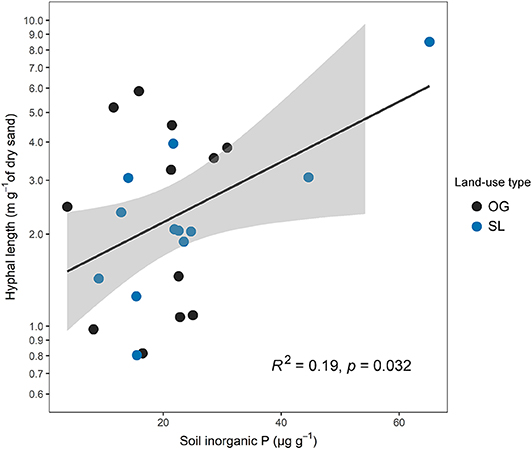
Figure 6. Relationship between mycelial production (hyphal length) and soil inorganic P concentrations across old-growth (OG) and selectively logged (SL) lowland dipterocarp rainforest in Sabah, Borneo. Hyphal length values are shown on a log scale corresponding to statistical tests. This relationship was found to be non-significant when the data point with high values in both parameters (top-right) was excluded from analysis (F = 0.83, R2 = 0.04, p = 0.371).
Discussion
We did not find significant shifts in community structure of any mycelial fungal group between OG and SL forest (Figures 2A–D), despite clear differences in soil and vegetation properties (higher soil bulk density, proportion of pioneer trees, woody and total NPP, and lower LAI and canopy NPP in logged forest) and plot-level tree community composition, broadly in line with previous findings from these study sites (Riutta et al., 2018; Both et al., 2019). This finding contrasts with our hypothesis (H1) and recent surveys which found the compositions of bulk soil fungal communities in tropical Southeast Asian dipterocarp forest to be highly sensitive to logging (Kerfahi et al., 2014; Mcguire et al., 2015). Our results suggest that actively foraging fungi may be more resilient to disturbance than the wider fungal community in bulk soil. However, direct comparability with other studies may be affected by methodological differences in sampling related to community capture rates (Wallander et al., 2001; Ekblad et al., 2013; i.e. micro-environmental conditions inside in-growth bags potentially selecting for certain fungal species). We also highlight that the in-growth bag method recovers only active hyphae, therefore avoiding confounding effects of relic DNA on soil microbial community patterns that can be a concern in studies across environmental gradients (Lennon et al., 2018). The total number of ASVs detected was lower than found in other recent ITS amplicon sequencing studies of bulk soil fungal communities in the region, which may exceed 25,000 (Elias, unpublished data), which we similarly attribute to these reasons. We identified differences in other mycelial fungal attributes between OG and SL forest, including a significant increase in the relative abundances of AM and ErM fungi in SL forest. The vast majority of forest mycorrhizal reads comprised EcM fungi (Figure 3). The canopy of lowland rainforests of Borneo is typically dominated by species belonging to the Dipterocarpaceae, which are an EcM-associating family (Taylor and Alexander, 2005; Brearley, 2012). Although our finding may relate to presence of host tree species, high EcM relative to AM fungal relative abundance may also result from amplification bias associated with ITS primers (Hart et al., 2015). Although mean dipterocarp basal area (Table 2) and relative abundance of EcM fungi (Table 3; Figure 3) were lower in SL forest, we did not detect significant differences in either metric due to the high variability in both. We nevertheless consider the relative increase in abundances of AM and ErM fungi in SL forest (the only other mycorrhizal types identified; Figure 3) to indicate increasing evenness in mycorrhizal types and diminished dominance of EcM fungi. This pattern reflects differences in vegetation indicated by the increased proportion of non-dipterocarp pioneer species in SL forest, which typically form arbuscular mycorrhizas (Mcguire et al., 2008). This shift may have implications for nutrient and C cycling, as recent correlative studies have suggested EcM- rather than AM-dominated communities may promote soil C storage on a global scale (Averill et al., 2014) through their ability to access N and P (Liu et al., 2018) from organic sources, potentially competing with free-living soil decomposers requiring N for the breakdown of organic matter (Averill et al., 2014). An increase in relative abundances of AM fungi may, therefore, have implications for the release of C from the soil as N becomes more available for soil saprotrophs. However, our analysis revealed no relationship between EcM fungal relative abundance and soil C concentration. It is possible that patterns reported in other studies (e.g. Averill et al., 2014) reflect much longer timescales in dominance of a particular mycorrhizal type than seen in our study. Alterations in soil C-cycling processes in SL dipterocarp forest have been observed, although as litter decomposition has been found to be slowed in SL forest due to subtle changes in forest microclimatic conditions (Both et al., 2017), the overall consequences for biogeochemical cycling and ecosystem functions are unclear.
Overall fungal community structure within in-growth bags was significantly different between OP plantation and OG and SL forests, and the impact of OP as a driver of differences over land-use types for saprotrophic, mycorrhizal and pathogenic mycelial fungal communities were also observed (Figures 2A–D), which corroborates the effects of forest conversion on bulk soil fungal communities (Mcguire et al., 2015). Mycorrhizal richness was also strongly affected (Figure 4A), with much lower values in oil palm relative to forest plots. As we found no differences between OG and SL forest, overall mycelial fungal communities appear to follow a similar pattern to soil bacterial communities, which have been shown to be generally unaffected by logging but distinctly different in oil palm plantation (Lee-Cruz et al., 2013; Tripathi et al., 2016).
In contrast to expectations (H1), mycelial production did not significantly differ between OG and SL forest, but was significantly less in OP relative to both forest land-use types (Figure 4B). We also found that hyphal length did not correspond to shifts in mycelial community attributes, soil properties and vegetation community between SL and OG forest, which may reflect resilience of some soil functions to selective logging (e.g. enzyme activity; Mcguire et al., 2015). The resilience of both mycelial production and relative abundance of mycelial EcM fungi implies their extramatrical mycelium may be largely retained in SL forest, when individual hosts remain. Mycorrhizal mycelial networks are crucial for tree seedling establishment (Nara, 2006), and may facilitate interplant exchange of resources with trees sharing the same mycorrhizal partnerships (Gorzelak et al., 2015). Our findings indicate implications for the potential restoration of these degraded forests, as EcM mycelial networks vital for supporting dipterocarp recruitment and survival may remain even after individuals of this family have been removed. Although basal area was significantly highest in OP, we found significantly lower mean root NPP in OP relative to SL (Table 2). There was also a complete removal of dipterocarp species through forest conversion to agricultural plantation. Furthermore, although canopy NPP was not measured in the OP site, significantly lower soil organic layer depth (Table 2) may indicate reduced litter inputs – especially as dead palm fronds are typically removed and collected in localized areas as standard in OP. This suggests a combination of a lack of EcM fungi, due to replacement by AM-associating oil palm species (Phosri et al., 2010), diminished belowground allocation of C by trees and reduced organic matter inputs may be down-regulating mycelial production rates in this land-use type. This corresponds to a ten-fold increase in the availability of limiting nutrients (inorganic P; Table 2) in OP due to the use of fertilizer. As the OP plantation was established after the selective-logging of these forests, lower hyphal length values, mycorrhizal abundance and richness appears to result from magnitude of EcM host removal rather than time since disturbance. The significant reduction in the soil (mycorrhizal) mycelium through the extreme disturbance of forest conversion represents a substantial barrier to the restoration of these systems.
Across OG and SL forest, mycelial fungal community attributes significantly varied with vegetation and soil properties (H2), but did not correspond with mycelial production (H3). Soil microbial community assemblage, particularly fungi, have been shown to correlate with aboveground plant taxonomic and phylogenetic structure in addition to local soil properties (Barberán et al., 2015). Soil pH was a significant predictor of community composition for all fungal groups, especially mycorrhizal and pathogenic fungi, a pattern observed across large scales in temperate ecosystems (Dupont et al., 2016). Mycorrhizal fungal community structure was surprisingly not affected by soil texture, previously found to be a strong predictor of EcM communities in Bornean lowland dipterocarp rainforest (Essene et al., 2017). It is possible that the use of sand inside in-growth bags masked the influence of bulk soil texture. Tree and mycorrhizal fungal community structure were significantly correlated at the plot-level (Figure 5), likely driven by the significant relationship between mycorrhizal fungal community dissimilarity and total dipterocarp basal area (Table 5). Previous studies in Bornean lowland dipterocarp rainforest have shown EcM fungal communities to be related to dipterocarp species assemblage in addition to strong effects of soil properties, suggesting dipterocarp species distributions across different soil types to be mediated by assemblage of their mycorrhizal partners (Essene et al., 2017). However, we found no significant correlation between mycorrhizal fungal and dipterocarp community dissimilarities, indicating overall abundance rather than composition of dipterocarps is more important in structuring actively foraging mycorrhizal fungal communities in the present study.
Mycelial productivity was significantly positively related to soil inorganic P (Figure 6), although mycelial biomass has previously been shown to be greater in more P-deficient forests (Potila et al., 2009). However, in some cases the proliferation of mycorrhizal hyphae into nutrient ‘hotspots' has been observed both in laboratory experiments and natural systems (Ekblad et al., 2013), whilst the role of inorganic P in controlling rates of mycelial production has been demonstrated in forests using hyphal in-growth bags augmented with mineral P, and field manipulations using fertilizer applications (Hagerberg et al., 2003; Potila et al., 2009; Ekblad et al., 2013; Camenzind et al., 2016). In the current study, the tenuous nature of this relationship (being mainly driven by one sampling point in SL; Figure 6) limits interpretation of underlying mechanisms, and should be approached with caution. Further study is required to confirm this link, which may be achieved with alterations in sampling design discussed below.
Some limitations must be recognized with respect to the interpretation of the results in this study. Primarily, soil sampling was conducted 12-14 months before installation and 18-20 months before collection of hyphal in-growth bags. Any alterations in soil properties arising from climatic factors, vegetation dynamics (i.e. regeneration of SL forest) or OP management during this period will not have been accounted for. Unlike rainforests in other tropical regions, the forests of Borneo do not experience a defined wet or dry season (Richards, 1996; Dykes, 1997) reflected in relatively stable canopy litter production throughout the year (Kumagai and Porporato, 2012). Differences in time of year sampled would not be expected to have a pronounced effect (i.e. due to coupled precipitation-driven seasonal dynamics in litter inputs, soil conditions and soil microbial communities observed other tropical systems; Buscardo et al., 2018), although the potential influence of supra-annual climatic and phenological variation (Itioka and Yamauti, 2004) and irregular inter-annual dry spells (Walsh and Newbery, 1999; Kumagai et al., 2005) should be noted. Furthermore, soil data represent bulk soil conditions at the subplot scale (10 × 10 m), rather than those within bags to which fungi were directly exposed. The combined temporal and spatial decoupling of soil and fungal community-productivity data may underlie the general lack of associations found between soil characteristics and mycelial productivity (also problematic for confidence in relationships observed, i.e. inorganic P; Figure 6) or fungal community attributes (e.g. little overall variation in community dissimilarities explained by soil factors; Table 5). Future studies should aim to concurrently sample for soil physicochemical properties and soil fungal communities and mycelial productivity (during installation, incubation and harvesting of hyphal-ingrowth bags). Corresponding molecular analysis of bulk soil fungal communities for comparison with in-growth bags, in tandem with a temporally coupled approach, would help to confirm the reciprocal mechanistic relationships between soil properties and fungal community-productivity patterns.
The present study analyzed data from 27 composite samples from 9 plots distributed across three land-use types at the landscape scale (Supplementary Information 1). Although composite samples comprised material from 72 individual in-growth bags to control for local variation, an unexpectedly high level of variability in fungal community attributes and mycelial productivity was observed across land-uses (particularly within the two forest types; Figure 4B). This variability (Table 3) makes it difficult to ascertain effects (or lack thereof) of management, and future surveys should incorporate greater plot-level replication in light of these findings. Similarly, site effects cannot be excluded from differences detected in fungal community attributes and mycelial productivity between OP and forest samples, as replication was restricted to only one 1 ha plot in this land-use type. While consistency with previous studies in peninsular Malaysia and Borneo (e.g. major reductions in EcM relative abundance in OP relative to OG and SL dipterocarp rainforest; Kerfahi et al., 2014; Mcguire et al., 2015) suggests findings to be representative of the broad impacts of forest conversion to OP on actively-foraging fungal communities, caution should be exercised in their interpretation.
The in-growth bag method has been found to adequately represent mycorrhizal mycelial productivity, as demonstrated by δ13C (Wallander et al., 2001) and molecular (Kjøller, 2006) analyses of bags in EcM-dominated systems. However, our results indicate that this may not be the case in tropical forest, with the largest proportion of overall reads belonging to ASVs identified as saprotrophs. As such, hyphae measured cannot be directly attributed to one fungal group or mycorrhizal type in this study. Incorporation of biomarker analyses to partition biomass by fungal types, or the inclusion of additional measures to reduce saprotrophic mycelial ingress are needed improve understanding of mycorrhizal productivity alone (Wallander et al., 2013). We found a high level of variability in mycelial fungal community dissimilarities across forest plots. Our analysis focused on the topsoil, as this is where the vast majority of nutrient turnover occurs. Here, fine-scale environmental heterogeneity can drive greater spatio-temporal fungal community variation compared to underlying mineral soil where communities may differ (Bahram et al., 2015). Future studies considering effects of forest degradation on mycelial fungal communities and productivity at different soil depths may identify different patterns along the soil profile.
In conclusion, selective logging did not significantly shift mycelial fungal community structure or productivity from OG forest, suggesting mycelial fungal communities and function may be relatively resilient to forest degradation compared to fungi in bulk soil. Results indicate the extramatrical EcM mycelium to be largely retained in selectively logged forest, with positive implications for potential restoration of dipterocarp forest by providing existing mycorrhizal networks for tree seedling establishment. However, SL forest was associated with higher relative abundances of AM and ErM fungi relative to OG, which may have consequences for soil C cycling and storage in lowland rainforests in Borneo over longer timescales than those reported here. Mycelial production was not related to any fungal community attributes, and broadly independent of vegetation, soil and environmental characteristics apart from a weak positive relationship with soil inorganic P concentration. In contrast, land conversion to oil palm plantation was shown to have a significant effect on overall, saprotrophic, mycorrhizal, and pathogenic mycelial fungal community structures, reducing mycorrhizal relative abundance and diversity. Mycelial production was significantly lower in oil palm relative to forest, corresponding to soil inorganic P concentrations of an order of magnitude greater under this land-use type. The impact of forest conversion on fungal community attributes and mycelial productivity may have wide repercussions for nutrient dynamics, plant-soil interactions and restoration potential over large scales as oil palm plantation has rapidly become a major land-use across Southeast Asia.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher. The molecular datasets generated for this study can be found in the European Nucleotide Archive with study accession no. PRJEB357.
Author Contributions
SR, DE, DJ, SB, NPM, and NO contributed to the initial conceptual design of the study. SR and DE established study plots and collected soil samples and data in the field. TG conducted DNA sequencing analysis and bioinformatics to generate molecular dataset. RG and TG provided conceptual approach to statistical analysis of microbial communities. SR, DJ, DE, and NPM contributed to hyphal extraction method. DE, NPM, and NM conducted soil analysis and provided soil characteristics dataset. SB provided tree community and functional trait dataset. TR provide tree productivity dataset. SR conducted statistical analysis and wrote the first draft of the manuscript. DE, SB, TR, NPM, RG, NO, DJ, and TG contributed writing to the manuscript. All authors provided critical revision of intellectual content, and approved the manuscript for submission.
Funding
This study was funded through the UK Natural Environment Research Council (NERC) Human Modified Tropical Forests programme (grant number NE/K016377/1) and Sime Darby Foundation funding to the SAFE project. This publication is a contribution from the NERC Biodiversity and Land-use Impacts on Tropical Ecosystem Function (BALI) consortium. DJ receives partial support from the N8 Agrifood programme.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We express our gratitude to the Sabah Biodiversity Council (SaBC), Yayasan Sabah (Sabah Foundation), the Danum Valley and Maliau Basin Management Committees, Glen Reynolds and the South East Asia Rainforest Research Partnership (SEARRP), and Rob Ewers for support and granting permission to access field sites [SaBC Access License no. JKM/MBS.1000-2/2 JLD.3 (173)]. We are extremely grateful for support from Laura Kruitbos, Unding Jami, and all research assistants in Sabah, without whom this study would not have been possible.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/ffgc.2020.00064/full#supplementary-material
References
Allen, S. E. (1989). Chemical Analysis of Ecological Materials, 2nd Edition, Oxford; London: Blackwell Scientific Publications.
Anderson, J. M., and Ingram, J. S. I. (1993). Tropical Soil Biology and Fertility: A Handbook of Methods, Wallingford: CAB International.
Appanah, S., and Turnbull, J. M. (1998). A Review of Dipterocarps: Taxonomy, Ecology and Silviculture. Available online at: http://www.cifor.org/publications/pdf_files/Books/Dipterocarps.pdf.
Averill, C., Turner, B. L., and Finzi, A. C. (2014). Mycorrhiza-mediated competition between plants and decomposers drives soil carbon storage. Nature 505, 543–545. doi: 10.1038/nature12901
Babikova, Z., Gilbert, L., Bruce, T. J. A., Birkett, M., Caulfield, J. C., Woodcock, C., et al. (2013). Underground signals carried through common mycelial networks warn neighbouring plants of aphid attack. Ecol. Lett. 16, 835–843. doi: 10.1111/ele.12115
Baccini, A., Walker, W., Carvalho, L., Farina, M., Sulla-Menashe, D., and Houghton, R. A. (2017). Tropical forests are a net carbon source based on aboveground measurements of gain and loss. Science, 358:230. doi: 10.1126/science.aam5962
Bahram, M., Peay, K. G., and Tedersoo, L. (2015). Local-scale biogeography and spatiotemporal variability in communities of mycorrhizal fungi. New Phytol. 205, 1454–1463. doi: 10.1111/nph.13206
Bakker, M., Delerue, F., Andreasson, F., Ngao, J., Dannoura, M., Zeller, B., et al. (2015). Hyphal growth in ingrowth mesh bags in fagus sylvatica, quercus petraea and pinus pinaster stands in france. Eur. J. Soil Biol. 70, 111–117. doi: 10.1016/j.ejsobi.2015.08.003
Barberán, A., Mcguire, K. L., Wolf, J. A., Jones, F. A., Wright, S. J., Turner, B. L., et al. (2015). Relating belowground microbial composition to the taxonomic, phylogenetic, and functional trait distributions of trees in a tropical forest. Ecol. Lett. 18, 1397–1405. doi: 10.1111/ele.12536
Barto, E. K., Weidenhamer, J. D., Cipollini, D., and Rillig, M. C. (2012). Fungal superhighways: do common mycorrhizal networks enhance below ground communication? Trends Plant Sci. 17, 633–637. doi: 10.1016/j.tplants.2012.06.007
Bates, D., Mächler, M., Bolker, B., and Walker, S. (2015). Fitting linear mixed-effects models using LME4. J. Statis. Softw. 67, 1–48. doi: 10.18637/jss.v067.i01
Berruti, A., Desirò, A., Visentin, S., Zecca, O., and Bonfante, P. (2017). ITS fungal barcoding primers versus 18S AMF-specific primers reveal similar AMF-based diversity patterns in roots and soils of three mountain vineyards. Environ. Microbiol. Rep. 9, 658–667. doi: 10.1111/1758-2229.12574
Bever, J. D., Dickie, I. A., Facelli, E., Facelli, J. M., Klironomos, J., Moora, M., et al. (2010). Rooting theories of plant community ecology in microbial interactions. Trends Ecol. Evol. 25, 468–478. doi: 10.1016/j.tree.2010.05.004
Blanchet, F. G., Legendre, P., and Borcard, D. (2008). Forward selection of explanatory variables. Ecology 89, 2623–2632. doi: 10.1890/07-0986.1
Boddington, C. L., Bassett, E. E., Jakobsen, I., and Dodd, J. C. (1999). Comparison of techniques for the extraction and quantification of extra-radical mycelium of arbuscular mycorrhizal fungi in soils. Soil Biol. Biochem. 31, 479–482. doi: 10.1016/S0038-0717(98)00145-X
Both, S., Elias, D. M. O., Kritzler, U. H., Ostle, N. J., and Johnson, D. (2017). Land use not litter quality is a stronger driver of decomposition in hyperdiverse tropical forest. Ecol. Evol. 7, 9307–9318. doi: 10.1002/ece3.3460
Both, S., Riutta, T., Paine, C. E. T., Elias, D. M. O., Cruz, R. S., Jain, A., et al. (2019). Logging and soil nutrients independently explain plant trait expression in tropical forests. New Phytol. 221:1853. doi: 10.1111/nph.15444
Bray, R. H., and Kurtz, L. T. (1945). Determination of total organic and available forms of phosphorus in soils. Soil Sci. 59, 39–46. doi: 10.1097/00010694-194501000-00006
Brearley, F. Q. (2012). Ectomycorrhizal associations of the dipterocarpaceae. Biotropica, 44, 637–648. doi: 10.1111/j.1744-7429.2012.00862.x
Bryan, J. E., Shearman, P. L., Asner, G. P., Knapp, D. E., Aoro, G., and Lokes, B. (2013). Extreme differences in forest degradation in borneo: comparing practices in sarawak, sabah, and brunei. PloS ONE 8:e69679. doi: 10.1371/journal.pone.0069679
Buscardo, E., Geml, J., Schmidt, S., Freitas, H., Da Cunha, H., and Nagy, L. (2018). Spatio-temporal dynamics of soil bacterial communities as a function of amazon forest phenology. Sci. Rep. 8:4382. doi: 10.1038/s41598-018-22380-z
Cairney, J. W. G. (2012). Extramatrical mycelia of ectomycorrhizal fungi as moderators of carbon dynamics in forest soil. Soil Biol. Biochem. 47, 198–208. doi: 10.1016/j.soilbio.2011.12.029
Callahan, B. J., Mcmurdie, P. J., Rosen, M. J., Han, A. W., Johnson, A. J. A., and Holmes, S. P. (2016). Dada2: high-resolution sample inference from illumina amplicon data. Nat. Methods 13, 581–583. doi: 10.1038/nmeth.3869
Camenzind, T., Papathanasiou, H. J., Forster, A., Dietrich, K., Hertel, D., Homeier, J., et al. (2016). Increases in soil aggregation following phosphorus additions in a tropical premontane forest are not driven by root and arbuscular mycorrhizal fungal abundances. Front. Earth Sci. 3:89. doi: 10.3389/feart.2015.00089
Chen, W., Koide, R. T., Adams, T. S., Deforest, J. L., Cheng, L., and Eissenstat, D. M. (2016). Root morphology and mycorrhizal symbioses together shape nutrient foraging strategies of temperate trees. Proc. Natl. Acad. Sci. U.S.A. 113:8741. doi: 10.1073/pnas.1601006113
Clemmensen, K. E., Finlay, R. D., Dahlberg, A., Stenlid, J., Wardle, D. A., and Lindahl, B. D. (2015). Carbon sequestration is related to mycorrhizal fungal community shifts during long-term succession in boreal forests. New Phytol. 205, 1525–1536. doi: 10.1111/nph.13208
Cortois, R., Schröder-Georgi, T., Weigelt, A., van der Putten, W. H., and De Deyn, G. B. (2016). Plant-soil feedbacks: role of plant functional group and plant traits. J. Ecol. 104, 1608–1617. doi: 10.1111/1365-2745.12643
Drigo, B., Anderson, I. C., Kannangara, G. S. K., Cairney, J. W. G., and Johnson, D. (2012). Rapid incorporation of carbon from ectomycorrhizal mycelial necromass into soil fungal communities. Soil Biol. Biochem. 49, 4–10. doi: 10.1016/j.soilbio.2012.02.003
Dufrêne, M., and Legendre, P. (1997). Species assemblages and indicator species:the need for a flexible asymmetrical approach. Ecol. Monogr. 67, 345–366. doi: 10.1890/0012-9615(1997)067[0345:SAAIST]2.0.CO;2
Dupont, A. Ö. C., Griffiths, R. I., Bell, T., and Bass, D. (2016). Differences in soil micro-eukaryotic communities over soil ph gradients are strongly driven by parasites and saprotrophs. Environ. Microbiol. 18, 2010–2024. doi: 10.1111/1462-2920.13220
Dykes, A. P. (1997). Rainfall interception from a lowland tropical rainforest in brunei. J. Hydrol. 200, 260–279. doi: 10.1016/S0022-1694(97)00023-1
Ek, H., Sjögren, M., Arnebrant, K., and Söderström, B. (1994). Extramatrical mycelial growth, biomass allocation and nitrogen uptake in ectomycorrhizal systems in response to collembolan grazing. Appl. Soil Ecol. 1, 155–169. doi: 10.1016/0929-1393(94)90035-3
Ekblad, A., Wallander, H., Godbold, D., Cruz, C., Johnson, D., Baldrian, P., et al. (2013). The production and turnover of extramatrical mycelium of ectomycorrhizal fungi in forest soils: role in carbon cycling. Plant Soil 366, 1–27. doi: 10.1007/s11104-013-1630-3
Elias, D. M. O., Mcnamara, N. P., Ostle, N. J., and Majalap-Lee, N. (2018). Soil Properties across Primary Forest, Logged Forest and Oil Palm Plantation in Sabah, Malaysia: NERC Environmental Information Data Centre.
Emmett, B. A., Frogbrook, Z. L., Chamberlain, P. M., Griffiths, R., Pickup, R., Poskitt, J., et al. (2008). Countryside Survey Technical Report No.03/07. Available online at: http://www.countrysidesurvey.org.uk/sites/default/files/CS_UK_2007_TR3%20-%20Soils%20Manual.pdf
Essene, A. L., Shek, K. L., Lewis, J. D., Peay, K. G., and Mcguire, K. L. (2017). Soil type has a stronger role than dipterocarp host species in shaping the ectomycorrhizal fungal community in a bornean lowland tropical rain forest. Front. Plant Sci. 8:1828. doi: 10.3389/fpls.2017.01828
Ewers, R. M., Didham, R. K., Fahrig, L., Ferraz, G., Hector, A., Holt, R. D., et al. (2011). A large-scale forest fragmentation experiment: the stability of altered forest ecosystems project. Philos. Trans. Roy. Soc. B 366, 3292–3302. doi: 10.1098/rstb.2011.0049
Finlay, R. D. (2008). Ecological aspects of mycorrhizal symbiosis: with special emphasis on the functional diversity of interactions involving the extraradical mycelium. J. Exp. Bot. 59, 1115–1126. doi: 10.1093/jxb/ern059
Fisher, B., Edwards, D. P., Giam, X., and Wilcove, D. S. (2011). The high costs of conserving southeast asia's lowland rainforests. Front. Ecol. Environ. 9:79. doi: 10.1890/100079
Fisher, J., Malhi, Y., Torres, I., Metcalfe, D., Van De Weg, M., Meir, P., et al. (2013). Nutrient limitation in rainforests and cloud forests along a 3,000-m elevation gradient in the peruvian andes. Oecologia 172, 889–902. doi: 10.1007/s00442-012-2522-6
Fitzherbert, E. B., Struebig, M. J., Morel, A., Danielsen, F., Brühl, C. A., Donald, P. F., et al. (2008). How will oil palm expansion affect biodiversity? Trends Ecol. Evol. 23, 538–545. doi: 10.1016/j.tree.2008.06.012
Gaveau, D. L. A., Sheil, D., Husnayaen, S. M. A., Arjasakusuma, S., Ancrenaz, M., Pacheco, P., et al. (2016). Rapid conversions and avoided deforestation: examining four decades of industrial plantation expansion in borneo. Sci. Rep. 6:32017. doi: 10.1038/srep32017
Gaveau, D. L. A., Sloan, S., Molidena, E., Yaen, H., Sheil, D., Abram, N. K., et al. (2014). Four decades of forest persistence, clearance and logging on borneo. PloS ONE 9:e101654. doi: 10.1371/journal.pone.0101654
Gorzelak, M. A., Asay, A. K., Pickles, B. J., and Simard, S. W. (2015). Inter-plant communication through mycorrhizal networks mediates complex adaptive behaviour in plant communities. AoB plants 7:plv050. doi: 10.1093/aobpla/plv050
Hagenbo, A., Kyaschenko, J., Clemmensen, K. E., Lindahl, B. D., and Fransson, P. (2018). Fungal community shifts underpin declining mycelial production and turnover across a pinus sylvestris chronosequence. J. Ecol. 106, 490–501. doi: 10.1111/1365-2745.12917
Hagerberg, D., Thelin, G., and Wallander, H. (2003). The production of ectomycorrhizal mycelium in forests: relation between forest nutrient status and local mineral sources. Plant Soil 252, 279–290. doi: 10.1023/A:1024719607740
Hansen, M. C., Potapov, P. V., Moore, R., Hancher, M., Turubanova, S. A., Tyukavina, A., et al. (2013). High-Resolution global maps of 21st-century forest cover change. Science 342:850. doi: 10.1126/science.1244693
Hanssen, J. F., Thingstad, T. F., and Goksøyr, J. (1974). Evaluation of hyphal lengths and fungal biomass in soil by a membrane filter technique. Oikos, 25, 102–107. doi: 10.2307/3543552
Hart, M. M., Aleklett, K., Chagnon, P. L., Egan, C., Ghignone, S., Helgason, T., et al. (2015). Navigating the labyrinth: a guide to sequence-based, community ecology of arbuscular mycorrhizal fungi. New Phytol. 207, 235–247. doi: 10.1111/nph.13340
Hartmann, M., Niklaus, P. A., Zimmermann, S., Schmutz, S., Kremer, J., Abarenkov, K., et al. (2013). Resistance and resilience of the forest soil microbiome to logging-associated compaction. ISME J. 8, 226–244. doi: 10.1038/ismej.2013.141
Hazebroek, H. P., Adlin, T. Z., and Sinun, W. (2004). Maliau Basin: Sabah's Lost World. Kota Kinabalu: Natural History Publications (Borneo).
Ihrmark, K., Bodeker, I. T. M., Cruz-Martinez, K., Friberg, H., Kubartova, A., Schenck, J., et al. (2012). New primers to amplify the fungal its2 region - evaluation by 454-sequencing of artificial and natural communities. FEMS Microbiol. Ecol. 82, 666–677. doi: 10.1111/j.1574-6941.2012.01437.x
Itioka, T., and Yamauti, M. (2004). Severe drought, leafing phenology, leaf damage and lepidopteran abundance in the canopy of a bornean aseasonal tropical rain forest. J. Trop. Ecol. 20, 479–482. doi: 10.1017/S0266467404001658
Itoo, Z., and Reshi, Z. (2013). The multifunctional role of ectomycorrhizal associations in forest ecosystem processes. Bot. Rev. 79, 371–400. doi: 10.1007/s12229-013-9126-7
Johnson, D., Leake, J. R., Ostle, N., Ineson, P., and Read, D. J. (2002). In situ 13CO2 pulse-labelling of upland grassland demonstrates a rapid pathway of carbon flux from arbuscular mycorrhizal mycelia to the soil. New Phytol. 153, 327–334. doi: 10.1046/j.0028-646X.2001.00316.x
Kerfahi, D., Tripathi, B. M., Lee, J., Edwards, D. P., and Adams, J. M. (2014). the impact of selective-logging and forest clearance for oil palm on fungal communities in borneo. PLoS ONE 9:e111525. doi: 10.1371/journal.pone.0111525
Kjøller, R. (2006). Disproportionate abundance between ectomycorrhizal root tips and their associated mycelia. FEMS Microbiol. Ecol. 58, 214–224. doi: 10.1111/j.1574-6941.2006.00166.x
Kozich, J. J., Westcott, S. L., Baxter, N. T., Highlander, S. K., and Schloss, P. D. (2013). Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the miseq illumina sequencing platform. Appl. Environ. Microbiol. 79, 5112–5120. doi: 10.1128/AEM.01043-13
Kumagai, T. O., and Porporato, A. (2012). Drought-induced mortality of a bornean tropical rain forest amplified by climate change. J. Geophys. Res: Biogeosci. 117:G02032. doi: 10.1029/2011JG001835
Kumagai, T. O., Saitoh, T. M., Sato, Y., Takahashi, H., Manfroi, O. J., Morooka, T., et al. (2005). Annual water balance and seasonality of evapotranspiration in a bornean tropical rainforest. Agric. Forest Meteorol. 128, 81–92. doi: 10.1016/j.agrformet.2004.08.006
Lambers, H., Raven, J. A., Shaver, G. R., and Smith, S. E. (2008). Plant nutrient-acquisition strategies change with soil age. Trends Ecol Evol. 23, 95–103. doi: 10.1016/j.tree.2007.10.008
Landon, J. R. (1984). Booker Tropical Soil Manual: A Handbook for Soil Survey and Agricultural Land Evaluation in the Tropics and Subtropics, London: Booker Agriculture International.
Lee-Cruz, L., Edwards, D. P., Tripathi, B. M., and Adams, J. M. (2013). Impact of logging and forest conversion to oil palm plantations on soil bacterial communities in borneo. Appl. Environ. Microbiol. 79, 7290–7297. doi: 10.1128/AEM.02541-13
Legendre, P., and Borcard, D. (2018). Box-cox-chord transformations for community composition data prior to beta diversity analysis. Ecography 41, 1820–1824. doi: 10.1111/ecog.03498
Lekberg, Y., Vasar, M., Bullington, L. S., Sepp, S. K., Antunes, P. M., Bunn, R., et al. (2018). More bang for the buck? Can arbuscular mycorrhizal fungal communities be characterized adequately alongside other fungi using general fungal primers? New Phytol. 220, 971–976. doi: 10.1111/nph.15035
Lennon, J. T., Muscarella, M. E., Placella, S. A., and Lehmkuhl, B. K. (2018). How, when, and where relic DNA affects microbial diversity. mBio 9:e00637-18. doi: 10.1128/mBio.00637-18
Lenth, R. S., Henrik L.ove, J., Buerkner, P., and Herve, M. (2019). Estimated Marginal Means, Aka Least-Squares Means. Available online at: https://cran.r-project.org/package=emmeans
Lindahl, B. D., Ihrmark, K., Boberg, J., Trumbore, S. E., Högberg, P., Stenlid, J., et al. (2007). Spatial separation of litter decomposition and mycorrhizal nitrogen uptake in a boreal forest. New Phytol. 173, 611–620. doi: 10.1111/j.1469-8137.2006.01936.x
Liu, X., Burslem, D. F. R. P., Taylor, J. D., Taylor, A. F. S., Khoo, E., Majalap-Lee, N., et al. (2018). Partitioning of soil phosphorus among arbuscular and ectomycorrhizal trees in tropical and subtropical forests. Ecol. Lett. 21, 713–723. doi: 10.1111/ele.12939
Luke, S. (2017). Evaluating significance in linear mixed-effects models in R. Behav. Res. Methods 49, 1494–1502. doi: 10.3758/s13428-016-0809-y
Majdi, H., Truus, L., Johansson, U., Nylund, J.-E., and Wallander, H. (2008). Effects of slash retention and wood ash addition on fine root biomass and production and fungal mycelium in a norway spruce stand in sw sweden. Forest Ecol. Manag. 255, 2109–2117. doi: 10.1016/j.foreco.2007.12.017
Malhi, Y., Doughty, C. E., Goldsmith, G. R., Metcalfe, D. B., Girardin, C. A. J., Marthews, T. R., et al. (2015). The linkages between photosynthesis, productivity, growth and biomass in lowland amazonian forests. Glob. Change Biol. 21, 2283–2295. doi: 10.1111/gcb.12859
Marsh, C. W., and Greer, A. G. (1992). Forest land-use in sabah, malaysia: an introduction to danum valley. Philos. Trans. Roy. Soc. B Biol. Sci. 335, 331–339. doi: 10.1098/rstb.1992.0025
Marthews, T., Riutta, T., Oliveras Menor, I., Urrutia, R., Moore, S., Metcalfe, D., et al. (2014). Measuring Tropical Forest Carbon Allocation and Cycling: A RAINFOR-GEM Field Manual for Intensive Census Plots (v3.0). Manual, Global Ecosystems Monitoring Network. Available online at: https://gem.tropicalforests.ox.ac.uk
Martinez Arbizu, P. (2019). Pairwiseadonis: Pairwise Multilevel Comparison Using Adonis. R Package Version 0.3. Available online at: https://github.com/pmartinezarbizu/pairwiseadonis
Mcguire, K., D'angelo, H., Brearley, F., Gedallovich, S., Babar, N., Yang, N., et al. (2015). Responses of soil fungi to logging and oil palm agriculture in southeast asian tropical forests. Microb. Ecol. 69, 733–747. doi: 10.1007/s00248-014-0468-4
Mcguire, K., Henkel, T., Granzow De La Cerda, I., Villa, G., Edmund, F., and Andrew, C. (2008). Dual mycorrhizal colonization of forest-dominating tropical trees and the mycorrhizal status of non-dominant tree and liana species. Mycorrhiza 18, 217–222. doi: 10.1007/s00572-008-0170-9
Mcmurdie, P. J, and Holmes, S. (2013). Phyloseq: an r package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 8:e61217. doi: 10.1371/journal.pone.0061217
Miller, W. P., and Miller, D. M. (1987). A micro-pipette method for soil mechanical analysis. Commun. Soil Sci. Plant Anal. 18, 1–15. doi: 10.1080/00103628709367799
Mueller, R. C., Rodrigues, J. L. M., Nüsslein, K., and Bohannan, B. J. M. (2016). Land use change in the amazon rain forest favours generalist fungi. Func. Ecol. 30, 1845–1853. doi: 10.1111/1365-2435.12651
Myers, N., Mittermeier, R. A., Mittermeier, C. G., Da Fonseca, G. A., and Kent, J. (2000). Biodiversity hotspots for conservation priorities. Nature, 403:853. doi: 10.1038/35002501
Nara, K. (2006). Ectomycorrhizal networks and seedling establishment during early primary succession. New Phytol. 169, 169–178. doi: 10.1111/j.1469-8137.2005.01545.x
Nasto, M. K., Alvarez-Clare, S., Lekberg, Y., Sullivan, B. W., Townsend, A. R., and Cleveland, C. C. (2014). Interactions among nitrogen fixation and soil phosphorus acquisition strategies in lowland tropical rain forests. Ecol. Lett. 17, 1282–1289. doi: 10.1111/ele.12335
Nguyen, N. H., Song, Z., Bates, S. T., Branco, S., Tedersoo, L., Menke, J., et al. (2016). Funguild: an open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 20, 241–248. doi: 10.1016/j.funeco.2015.06.006
Nilsson, L. O., and Wallander, H. (2003). Production of external mycelium by ectomycorrhizal fungi in a norway spruce forest was reduced in response to nitrogen fertilization. New Phytol. 158, 409–416. doi: 10.1046/j.1469-8137.2003.00728.x
Nottingham, A. T., Turner, B. L., Winter, K., Chamberlain, P. M., Stott, A., and Tanner, E. V. J. (2013). Root and arbuscular mycorrhizal mycelial interactions with soil microorganisms in lowland tropical forest. FEMS Microbiol. Ecol. 85, 37–50. doi: 10.1111/1574-6941.12096
Oksanen, J., Blanchet, F. G., Kindt, R., Legendre, P., O'hara, R. B., Simpson, G. L., et al. (2019). Vegan: Community Ecology Package. Available online at: https://cran.r-project.org/package=vegan
Orwin, K. H., Kirschbaum, M. U. F., St John, M. G., and Dickie, I. A. (2011). Organic nutrient uptake by mycorrhizal fungi enhances ecosystem carbon storage: a model-based assessment. Ecol. Lett. 14, 493–502. doi: 10.1111/j.1461-0248.2011.01611.x
Pan, Y., Birdsey, R. A., Fang, J., Houghton, R., Kauppi, P. E., Kurz, W. A., et al. (2011). A large and persistent carbon sink in the world's forests. Science 333, 988–993. doi: 10.1126/science.1201609
Phosri, C., Rodriguez, A., Sanders, I. R., and Jeffries, P. (2010). The role of mycorrhizas in more sustainable oil palm cultivation. Agric. Ecosyst. Environ. 135, 187–193. doi: 10.1016/j.agee.2009.09.006
Potila, H., Wallander, H., and Sarjala, T. (2009). Growth of ectomycorrhizal fungi in drained peatland forests with variable p and k availability. Int. J. Plant Soil Relat. 316, 139–150. doi: 10.1007/s11104-008-9766-2
Powers, R. P., and Jetz, W. (2019). Global habitat loss and extinction risk of terrestrial vertebrates under future land-use-change scenarios. Nat. Clim. Change 9, 323–329. doi: 10.1038/s41558-019-0406-z
R Core Team (2013). R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. Available online at: http://www.r-project.org/
Richards, P. W. (1996). The Tropical Rain Forest: An Ecological Study, Cambridge: Cambridge University Press.
Rillig, M. C., Aguilar-Trigueros, C. A., Bergmann, J., Verbruggen, E., Veresoglou, S. D., and Lehmann, A. (2015). Plant root and mycorrhizal fungal traits for understanding soil aggregation. New Phytol. 174, 1385–1388. doi: 10.1111/nph.13045
Riutta, T., Malhi, Y., Kho, L. K., Marthews, T. R., Huaraca Huasco, W., Khoo, M., et al. (2018). Logging disturbance shifts net primary productivity and its allocation in bornean tropical forests. Glob. Change Biol. 24, 2913–2928. doi: 10.1111/gcb.14068
Roberts, D. W. (2016). Ordination and Multivariate Analysis for Ecology. Available online at: http://ecology.msu.montana.edu/labdsv/r
Schneider, C. A., Rasband, W. S., and Eliceiri, K. W. (2012). NIH Image to Imagej: 25 Years of Image Analysis. Nat. Methods 9, 671–675. doi: 10.1038/nmeth.2089
Setäl,ä, H., Kulmala, P., Mikola, J., and Markkola, A. M. (1999). Influence of ectomycorrhiza on the structure of detrital food webs in pine rhizosphere. Oikos, 87, 113–122. doi: 10.2307/3547002
Shen, Q., Kirschbaum, M. U. F., Hedley, M. J., and Camps Arbestain, M. (2016). Testing an alternative method for estimating the length of fungal hyphae using photomicrography and image processing. PLoS ONE 11:e0157017. doi: 10.1371/journal.pone.0157017
Simpson, G. L., R Core Team, Bates, D. M., and Oksanen, J. (2019). Functions for Generating Restricted Permutations of Data. Available online at: https://github.com/gavinsimpson/permute
Sodhi, N. S., Koh, L. P., Clements, R., Wanger, T. C., Hill, J. K., Hamer, K. C., et al. (2010). Conserving southeast asian forest biodiversity in human-modified landscapes. Biol. Conserv. 143, 2375–2384. doi: 10.1016/j.biocon.2009.12.029
Taylor, A. F. S., and Alexander, I. (2005). The ectomycorrhizal symbiosis: life in the real world. Mycologist 19, 102–112. doi: 10.1017/S0269-915X(05)00303-4
Tennant, D. (1975). A test of a modified line intersect method of estimating root length. J. Ecol. 63, 995–1001. doi: 10.2307/2258617
Tripathi, B. M., Edwards, D. P., Mendes, L. W., Kim, M., Dong, K., Kim, H., and Adams, J. M. (2016). the impact of tropical forest logging and oil palm agriculture on the soil microbiome. Mol. Ecol. 25, 2244–2257. doi: 10.1111/mec.13620
van der Heijden, M. G. A., Bardgett, R. D., and van Straalen, N. M. (2008). The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 11, 296–310. doi: 10.1111/j.1461-0248.2007.01139.x
van der Putten, W., Bardgett, R. D., Bever, J. D., Bezemer, T. M., Casper, B., Fukami, T., et al. (2013). Plant-Soil feedbacks: the past, the present and future challenges. J. Ecol. 101, 265–276. doi: 10.1111/1365-2745.12054
Wallander, H., Ekblad, A., Godbold, D. L., Johnson, D., Bahr, A., Baldrian, P., et al. (2013). Evaluation of methods to estimate production, biomass and turnover of ectomycorrhizal mycelium in forests soils - a review. Soil Biol. Biochem. 57, 1034–1047. doi: 10.1016/j.soilbio.2012.08.027
Wallander, H., Nilsson, L. O., Hagerberg, D., and Bååth, E. (2001). Estimation of the biomass and seasonal growth of external mycelium of ectomycorrhizal fungi in the field. New Phytol. 151, 753–760. doi: 10.1046/j.0028-646x.2001.00199.x
Walsh, R. P. D., and Newbery, D. M. (1999). The ecoclimatology of danum, sabah, in the context of the world's rainforest regions, with particular reference to dry periods and their impact. Philos. Trans. Roy. Soc. B Biol. Sci. 354, 1869–1883. doi: 10.1098/rstb.1999.0528
Wardle, D., Bardgett, R. D., Klironomos, J., Setala, H., van der Putten, W., and Wall, D. (2004). Ecological linkages between aboveground and belowground biota. Sci. 304, 1629–1633. doi: 10.1126/science.1094875
Wei, T., and Simko, V. (2017). R Package “corrplot”: Visualization of a Correlation Matrix. Available online at: https://github.com/taiyun/corrplot
Wickham, H. (2016). ggplot2: Elegant Graphics for Data Analysis, New York, NY: Springer-Verlag. doi: 10.1007/978-3-319-24277-4_9
Wilcove, D. S., Giam, X., Edwards, D. P., Fisher, B., and Koh, L. P. (2013). Navjot's nightmare revisited: logging, agriculture, and biodiversity in southeast asia. Trends Ecol. Evol. 28, 531–540. doi: 10.1016/j.tree.2013.04.005
Wilson, G. W. T., Rice, C. W., Rillig, M. C., Springer, A., and Hartnett, D. C. (2009). Soil aggregation and carbon sequestration are tightly correlated with the abundance of arbuscular mycorrhizal fungi: results from long-term field experiments. Ecol. Lett. 12, 452–461. doi: 10.1111/j.1461-0248.2009.01303.x
Wurzburger, N., and Clemmensen, K. E. (2018). From mycorrhizal fungal traits to ecosystem properties - and back again. J. Ecol. 106, 463–467. doi: 10.1111/1365-2745.12922
Keywords: oil palm, selective logging, mycorrhiza, arbuscular, ectomycorrhizal, hyphae, inorganic P, ITS
Citation: Robinson SJB, Elias D, Johnson D, Both S, Riutta T, Goodall T, Majalap N, McNamara NP, Griffiths R and Ostle N (2020) Soil Fungal Community Characteristics and Mycelial Production Across a Disturbance Gradient in Lowland Dipterocarp Rainforest in Borneo. Front. For. Glob. Change 3:64. doi: 10.3389/ffgc.2020.00064
Received: 19 September 2019; Accepted: 04 May 2020;
Published: 26 June 2020.
Edited by:
Erika Buscardo, University of Brasilia, BrazilReviewed by:
Binu M. Tripathi, Korea Polar Research Institute, South KoreaTessa Camenzind, Freie Universität Berlin, Germany
Copyright © 2020 Robinson, Elias, Johnson, Both, Riutta, Goodall, Majalap, McNamara, Griffiths and Ostle. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Samuel J. B. Robinson, sjbrobinson@hotmail.co.uk
 Samuel J. B. Robinson
Samuel J. B. Robinson Dafydd Elias
Dafydd Elias David Johnson
David Johnson Sabine Both
Sabine Both Terhi Riutta
Terhi Riutta Tim Goodall7
Tim Goodall7  Robert Griffiths
Robert Griffiths Nick Ostle
Nick Ostle