Abstract
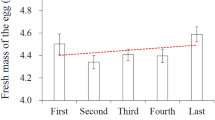
Egg composition varies both within and between clutches, and mothers are expected to alter their deposition of resources to the egg depending on environmental conditions and breeding strategies. Within-clutch variation in egg composition has been proposed to reflect an adaptive maternal strategy influencing sibling competition. In species with brood reduction, mothers should reinforce brood hierarchies due to hatching asynchrony and favour senior chicks by making first-laid eggs larger, richer in nutrients, with higher testosterone and carotenoid levels and lower corticosterone concentrations than last-laid eggs [parental favouritism hypothesis (PFH)]. Moreover, mothers that are of better quality and/or experience better feeding conditions during laying are expected to increase their deposition of resources to the egg, resulting in differences between clutches [investment hypothesis (IH)]. Several components may act together to provide an optimal reproductive strategy, but studies of variation in different egg components in the same egg are relatively rare. We analysed egg size, testosterone and corticosterone concentrations and carotenoids measured as yolk colour between and within clutches for the facultative siblicidal Black-legged Kittiwake Rissa tridactyla. First-laid eggs were larger, contained lower testosterone, higher yolk colour score, and similar corticosterone levels than last-laid eggs. Thus, only differences in egg size and yolk colour supported the PFH. We used within-clutch egg size dimorphism as an indicator of the quality of the mother or the feeding conditions during laying. In support of the IH, we found that mothers of better quality or that experienced better feeding conditions deposited more corticosterone into their eggs. High corticosterone levels may benefit nestlings when there is no brood reduction but high sibling competition is present. We found no support for the hypothesis that egg components are mutually adjusted to each other and we discuss the possible reasons for this.
Zusammenfassung
Die Zusammensetzung von Eiern variiert unabhängig von einander in der fakultative Geschwister-reduzierenden Dreizehenmöwe Rissa tridactyla
Die Zusammensetzung von Eiern variiert sowohl innerhalb als auch zwischen Gelegen. Es wird postuliert, dass Mütter die Zusammensetzung ihrer Eier variieren entsprechend der herrschenden Umweltbedingungen und ihrer Brutstrategie. Es wird angenommen, dass die Variation der Zusammensetzung von Eiern innerhalb von Gelegen eine adaptive elterliche Strategie darstellt, um die Konkurrenz zwischen Geschwistern zu beeinflussen. In Arten mit Brutreduktion könnten die Mütter die Hierarchie innerhalb einer Brut durch Schlüpfasynchronie und Bevorzugung des ältesten Kücken verstärken, indem das erstgelegte Ei mehr Nährstoffe, Testosteron und Karotenoide, aber weniger Corticosteron erhalten als das letzte Ei in einem Gelege (sog. parental favouritism hypothesis (PFH)). Zudem wird erwartet, dass Mütter in besserer Verfassung und/oder bei besseren Nahrungsumständen mehr Ressourcen in ihre Eier deponieren (sog. investment hypothesis (IH)). Mehrere Komponenten des Eies wirken möglicherweise zusammen, um eine optimale Brutstrategie darzustellen. Studien über die Variation von mehreren Eikomponenten im selben Ei sind selten. Wir analysierten die Variation in Eigröße, Testosteron, Corticosterone und Karotenoide (gemessen als Farbe des Eidotters) zwischen und innerhalb von Gelegen der fakultative Geschwister-reduzierenden Dreizehenmöwe Rissa tridactyla. Erstgelegte Eier waren größer, hatten mehr Testosteron, gelbere Dotter und ähnliche Mengen von Corticosteron verglichen mit letztgelegten Eiern. Damit unterstützen nur Unterschiede in Eigröße und Farbe des Eidotters die PFH. Wir benutzten den Größenunterschied zwischen Eiern des gleichen Geleges als Indikator der Nahrungssuchbedingungen der Mutter während der Eiablage. Wir fanden, dass Mütter in besserer Verfassung oder mit besseren Nahrungssuchbedingungen mehr Corticosteron in ihre Eier deponierten, was die IH unterstützt. Große Mengen von Corticosteron könnten für Kücken von Vorteil sein, wenn es trotz Geschwisterkonkurrenz keine Brutreduktion hat. Wir fanden keine Hinweise darauf, dass die Mengen verschiedener Eikomponenten aufeinander abgestimmt waren, und wir diskutieren mögliche Erklärungen.


Similar content being viewed by others
References
Angelier F, Wingfield JC, Weimerskirch H, Chastel O (2010) Hormonal correlates of individual quality in a long-lived bird: a test of the ‘corticosterone-fitness hypothesis’. Biol Lett 6:846–849
Beissinger SR, Stoleson SH (1997) Hatching asynchrony in birds. Trends Ecol Evol 12:112
Biard C, Surai PF, Moller AP (2007) An analysis of pre- and post-hatching maternal effects mediated by carotenoids in the blue tit. J Evol Biol 20:326–339
Blount JD, Houston DC, Moller AP (2000) Why egg yolk is yellow. Trends Ecol Evol 15:47–49
Blount JD, Surai PF, Nager RG, Houston DC, Moller AP, Trewby ML, Kennedy MW (2002) Carotenoids and egg quality in the lesser black-backed gull (Larus fuscus): a supplemental feeding study of maternal effects. Proc R Soc Lond B 269:29–36
Bogdanova MI, Nager RG (2008) Sex-specific costs of hatching last: an experimental study on herring gulls (Larus argentatus). Behav Ecol Sociobiol 62:1533–1541
Braun BM, Hunt GL (1984) Brood reduction in black-legged kittiwakes. Auk 100:469–473
Carey C (1996) Female reproductive energetics. In: Carey C (ed) Avian energetics and nutritional ecology. Chapman & Hall, New York, pp 324–374
Chin EH, Love OP, Verspoor JJ, Williams TD, Rowley K, Burness G (2009) Juveniles exposed to embryonic corticosterone have enhanced flight performance. Proc R Soc Lond B 276:499–505
Christians JK (2002) Avian egg size: variation within species and inflexibility within individuals. Biol Rev 77:1–26
Clark AB, Wilson DS (1981) Avian breeding adaptations—hatching asynchrony, brood reduction, and nest failure. Q Rev Biol 56:253–277
Coulson JC (1963) Egg size and shape in the kittiwake (Rissa tridactyla) and their use in estimating age composition of populations. Proc Zool Soc Lond 140:211–227
Crawley MJ (1993) GLIM for ecologists. Blackwell, Oxford
Crean AJ, Marshall DJ (2009) Coping with environmental uncertainty: dynamic bet hedging as a maternal effect. Philos Trans R Soc Lond B 364:1087–1096
Dentressangle F, Boeck L, Torres R (2008) Maternal investment in eggs is affected by male feet colour and breeding conditions in the blue-footed booby, Sula nebouxii. Behav Ecol Sociobiol 62:1899–1908
Dobush GR, Ankney CD, Lack D (1985) The effect of apparatus, extraction time, and solvent type on lipid extractions of Snow Geese. Can J Zool 63:1917–1920
Drummond H (2006) Dominance in vertebrate broods and litters. Q Rev Biol 81:3–32
Drummond H, Rodriguez C, Schwabl H (2008) Do mothers regulate facultative and obligate siblicide by differentially provisioning eggs with hormones? J Avian Biol 39:139–143
Eising CM, Eikenaar C, Schwabl H, Groothuis TGG (2001) Maternal androgens in black-headed gull (Larus ridibundus) eggs: consequences for chick development. Proc R Soc Lond B 268:839–846
Forbes S, Wiebe M (2010) Egg size and asymmetric sibling rivalry in red-winged blackbirds. Oecologia 163:361–372
Frederiksen M, Wanless S, Rothery P, Wilson LJ (2004) The role of industrial fisheries and oceanographic change in the decline of North Sea black-legged kittiwakes. J Appl Ecol 41:1129–1139
Gasparini J, Boulinier T, Gill VA, Gil D, Hatch SA, Roulin A (2007) Food availability affects the maternal transfer of androgens and antibodies into eggs of a colonial seabird. J Evol Biol 20:874–880
Gil D (2008) Hormones in avian eggs: physiology, ecology and behavior. Adv Stud Behav 38:337–398
Gil D, Ninni P, Lacroix A, De Lope F, Tirard C, Marzal A, Moller AP (2006) Yolk androgens in the barn swallow (Hirundo rustica): a test of some adaptive hypotheses. J Evol Biol 19:123–131
Greenwood JG (2003) Measuring sexual size dimorphism in birds. Ibis 145:E124–E126
Groothuis TGG, Schwabl H (2008) Hormone-mediated maternal effects in birds: mechanisms matter but what do we know of them? Philos Trans R Soc Lond B 363:1647–1661
Groothuis TGG, Eising CM, Dijkstra C, Muller W (2005a) Balancing between costs and benefits of maternal hormone deposition in avian eggs. Biol Lett 1:78–81
Groothuis TGG, Muller W, von Engelhardt N, Carere C, Eising C (2005b) Maternal hormones as a tool to adjust offspring phenotype in avian species. Neurosci Biobehav Rev 29:329–352
Groothuis TGG, Eising CM, Blount JD, Surai P, Apanius V, Dijkstra C, Muller W (2006) Multiple pathways of maternal effects in black-headed gull eggs: constraint and adaptive compensatory adjustment. J Evol Biol 19:1304–1313
Hargitai R, Csaba M, Miklos B, Gil D, Lopez-Rull I, Solymos E (2010) Eggshell characteristics and yolk composition in the common cuckoo Cuculus canorus: are they adapted to brood parasitism? J Avian Biol 41:177–185
Hayward LS, Wingfield JC (2004) Maternal corticosterone is transferred to avian yolk and may alter offspring growth and adult phenotype. Gen Comp Endocrinol 135:365–371
Hebert CE, Shutt JL, Ball RO (2002) Plasma amino acid concentrations as an indicator of protein availability to breeding Herring Gulls (Larus argentatus). Auk 119:185–200
Karadas F, Grammenidis E, Surai PF, Acamovic T, Sparks C (2006) Effects of carotenoids from lucerne, marigold and tomato on egg yolk pigmentation and carotenoid composition. Br Poult Sci 47:561–566
Kilpi M, Hillström L, Lindström K (1996) Egg-size variation and reproductive success in the herring gull Larus argentatus: adaptive or constrained size of the last egg? Ibis 138:212–217
Kim M, Furness RW, Nager RG (2010) Hatching asynchrony is constrained by parental nest attendance during laying. Behav Ecol Sociobiol 64:1087–1097
Kirkpatrick M, Lande R (1989) The evolution of maternal characters. Evolution 43:485–503
Kozlowski CP, Ricklefs RE (2010) Egg size and yolk steroids vary across the laying order in cockatiel clutches: a strategy for reinforcing brood hierarchies? Gen Comp Endocrinol 168:460–465
Krist M (2011) Egg size and offspring quality: a meta-analysis in birds. Biol Rev 86:692–716
Lack D (1947) The significance of clutch-size. Ibis 89:302–352
Lindström J (1999) Early development and fitness in birds and mammals. Trends Ecol Evol 14:343–348
Lipar JL (2001) Yolk steroids and the development of the hatching muscle in nestling European starlings. J Avian Biol 32:231–238
Lipar JL, Ketterson ED (2000) Maternally derived yolk testosterone enhances the development of the hatching muscle in the red-winged blackbird Agelaius phoeniceus. Proc R Soc Lond B 267:2005–2010
Lipar JL, Ketterson ED, Nolan V, Casto JM (1999) Egg yolk layers vary in the concentration of steroid hormones in two avian species. Gen Comp Endocrinol 115:220–227
Love OP, Williams TD (2008) Plasticity in the adrenocortical response of a free-living vertebrate: the role of pre- and post-natal developmental stress. Horm Behav 54:496–505
Love OP, Chin EH, Wynne-Edwards KE, Williams TD (2005) Stress hormones: a link between maternal condition and sex-biased reproductive investment. Am Nat 166:751–766
Love OP, Gilchrist HG, Bety J, Wynne-Edwards KE, Berzins L, Williams TD (2009) Using life-histories to predict and interpret variability in yolk hormones. Gen Comp Endocrinol 163:169–174
Maddox JD, Weatherhead PJ (2008) Egg size variation in birds with asynchronous hatching: is bigger really better? Am Nat 171:358–365
Maddox JD, Bowden RM, Weatherhead PJ (2008) Yolk testosterone and estradiol variation relative to clutch size, laying order and hatching asynchrony in common grackles. J Ornithol 149:643–649
Marshall DJ, Uller T (2007) When is a maternal effect adaptive? Oikos 116:1957–1963
McGraw KJ, Adkins-Regan E, Parker RS (2005) Maternally derived carotenoid pigments affect offspring survival, sex ratio, and sexual attractiveness in a colourful songbird. Naturwissenschaften 92:375–380
Metcalfe NB, Monaghan P (2001) Compensation for a bad start: grow now, pay later? Trends Ecol Evol 16:254–260
Mock DW, Parker GA (1997) The evolution of sibling rivalry. Oxford University Press, Oxford
Mousseau TA, Fox CW (1998) The adaptive significance of maternal effects. Trends Ecol Evol 13:403–407
Muck C, Nager RG (2006) The effect of laying and hatching order on the timing of hatching. Anim Behav 71:885–892
Nager RG (2006) The challenges of making eggs. Ardea 94:323–346
Nager RG, Monaghan P, Houston DC (2000) Within-Clutch trade-offs between the number and quality of eggs: experimental manipulations in gulls. Ecology 81:1339–1350
Nakagawa S, Cuthill IC (2007) Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol Rev 82:591–605
Navara KJ, Hill GE, Mendonca MT (2006a) Yolk androgen deposition as a compensatory strategy. Behav Ecol Sociobiol 60:392–398
Navara KJ, Badyaev AV, Mendonca MT, Hill GE (2006b) Yolk antioxidants vary with male attractiveness and female condition in the house finch (Carpodacus mexicanus). Physiol Biochem Zool 79:1098–1105
Nys Y (2000) Dietary carotenoids and egg yolk coloration—a review. Arch Geflügelkd 64:45–54
Okuliarova M, Sarnikova B, Rettenbacher S, Skrobanek P, Zeman M (2010) Yolk testosterone and corticosterone in hierarchical follicles and laid eggs of Japanese quail exposed to long-term restraint stress. Gen Comp Endocrinol 165:91–96
Paitz RT, Bowden RM, Casto JM (2011) Embryonica modulation of maternal steroids in European starlings (Sturnus vulgaris). Proc R Soc Lond B 278:99–106
Pilz KM, Smith HG, Sandell MI, Schwabl H (2003) Inter female variation in egg yolk androgen allocation in the European starling: do high-quality females invest more? Anim Behav 65:841–850
Pitala N, Ruuskanen S, Laaksonen T, Doligez B, Tschirren B, Gustafsson L (2009) The effects of experimentally manipulated yolk androgens on growth and immune function of male and female nestling collared flycatchers Ficedula albicollis. J Avian Biol 40:225–230
Poisbleau M, Demongin L, Trouve C, Quillfeldt P (2009) Maternal deposition of yolk corticosterone in clutches of southern rockhopper penguins (Eudyptes chrysocome chrysocome). Horm Behav 55:500–506
Rettenbacher S, Mostl E, Groothuis TGG (2009) Gestagens and glucocorticoids in chicken eggs. Gen Comp Endocrinol 164:125–129
Robertson AJ (2009) Hormonally mediated maternal effects in birds. Thesis, University of Glasgow
Romano M, Caprioli M, Ambrosini R, Rubolini D, Fasola M, Saino N (2008) Maternal allocation strategies and differential effects of yolk carotenoids on the phenotype and viability of yellow-legged gull (Larus michahellis) chicks in relation to sex and laying order. J Evol Biol 21:1626–1640
Royle NJ, Surai PF, Hartley IR (2001) Maternally derived androgens and antioxidants in bird eggs: complementary but opposing effects? Behav Ecol 12:381–385
Rubolini D, Romano M, Alquati AB, Saino N (2006) Early maternal, genetic and environmental components of antioxidant protection, morphology and immunity of yellow-legged gull (Larus michahellis) chicks. J Evol Biol 19:1571–1584
Safran RJ, Pilz KM, McGraw KJ, Correa SM, Schwabl H (2008) Are yolk androgens and carotenoids in barn swallow eggs related to parental quality? Behav Ecol Sociobiol 62:427–438
Saino N, Bertacche V et al (2002) Carotenoid concentration in barn swallow eggs is influenced by laying order, maternal infection and paternal ornamentation. Proc R Soc B Biol Sci 269(1501):1729–1733
Saino N, Romano M, Ferrari RP, Martinelli R, Moller AP (2005) Stressed mothers lay eggs with high corticosterone levels which produce low-quality offspring. J Exp Zool Part A 303A:998–1006
Sandell MI (2007) Exogenous testosterone increases female aggression in the European starling (Sturnus vulgaris). Behav Ecol Sociobiol 62:255–262
Schwabl H (1993) Yolk is a source of maternal testosterone for developing birds. Proc Natl Acad Sci USA 90:11446–11450
Schwabl H, Mock DW, Gieg JA (1997) A hormonal mechanism for parental favouritism. Nature 386:231
Slagsvold T, Sandvik J, Rofstad G, Lorentsen O, Husby M (1984) On the adaptive value of intraclutch egg-size variation in birds. Auk 101:685–697
Sockman KW, Sharp PJ, Schwabl H (2006) Orchestration of avian reproductive effort: an integration of the ultimate and proximate bases for flexibility in clutch size, incubation behaviour, and yolk androgen deposition. Biol Rev 81:629–666
Stenning MJ (1996) Hatching asynchrony, brood reduction and other rapidly reproducing hypotheses. Trends Ecol Evol 11:243–246
Surai PF, Speake BK, Wood NA, Blount JD, Bortolotti GR, Sparks NHC (2001) Carotenoid discrimination by the avian embryo: a lesson from wild birds. Comp Biochem Physiol B 128:743–750
Török J, Hargitai R, Hegyi G, Matus Z, Michl G, Peczely P, Rosivall B, Toth G (2007) Carotenoids in the egg yolks of collared flycatchers (Ficedula albicollis) in relation to parental quality, environmental factors and laying order. Behav Ecol Sociobiol 61:541–550
Vallarino A (2009) Sibling rivalry in black-legged Kittiwakes (Rissa tridactyla). VDM Dr Mueller, Saarbrücken
Verboven N, Evans NP, D’Alba L, Nager RG, Blount JD, Surai PF, Monaghan P (2005) Intra-specific interactions influence egg composition in the lesser black-backed gull (Larus fuscus). Behav Ecol Sociobiol 57:357–365
Vuilleum JP (1969) Roche yolk colour fan—an instrument for measuring yolk colour. Poult Sci 48:767–777
Williams TD (1994) Intraspecific variation in egg size and egg composition in birds—effects on offspring fitness. Biol Rev 69:35–59
Acknowledgments
We are grateful to the Mexican Council for Science and Technology (CONACyT) for funding the PhD of AV. Scottish Natural Heritage (SNH) provided the facilities and permits to carry out the field work on the Isle of May. We thank Liz Mackley and Stuart Murray for help with field work and Christine Whitelaw and Tony Robertson for help with the yolk hormone analyses. Comments from César González, Ton Groothuis and two anonymous referees improved the presentation of the manuscript. The work was carried out complying with the current laws of the UK under SNH permits and licences.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P. H. Becker.
Rights and permissions
About this article
Cite this article
Vallarino, A., Evans, N., Daunt, F. et al. Egg components vary independently of each other in the facultative siblicidal Black-legged Kittiwake Rissa tridactyla . J Ornithol 153, 513–523 (2012). https://doi.org/10.1007/s10336-011-0772-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-011-0772-4